RESEARCH ARTICLE
¹University of Tetova, Facultyof Food Technology and Nutrition, Tetova, North Macedonia
2St. Kliment OhridskiUniversity, Faculty of Technology and Technical Sciences-Veles
*Corresponding Author: Durim Alija ¹*, Universityof Tetova, Facultyof Food Technologyand Nutrition, Tetova, North Macedonia.
Citation: Durim Alija1*(2024), A The effect of adding different additives on acrylamide content and antioxidant activity of innovative functional cereal products, Dietary Nourishment and Food Processing Techniques (DNFPT)1(1), DOI: https://doi.org/10.64347/3064-7061/DNFPT.007
Copyright: © ( 2024), Durim Alija1*, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: February 24, 2024 | Accepted: April 29, 2024 | Published: May 01, 2024
Abstract
Acrylamide (AA) is a chemical pollutant that naturally forms in starchy food products during high temperature cooking, including frying, baking and also industrial processing, at +120°C and low humidity. The main chemical process that causes this is known as the Maillardreaction; it is the same reaction that "browns" food and affectsits taste. AA is formed from sugars and amino acids (mainly one called asparagine ASN) that are naturally present in many foods. AA is found in products such as crisps, chips, bread, biscuits and coffee. (Nematollahi et al., 2020). The health impact of acrylamide has been the subject of concern and research for several years. Research on laboratory animals has shown that exposure to high levels of acrylamide can cause a variety of adverse effects, including damage to the nervous system and an increased risk of some types of cancer. It is important to note that these studies often involve the administration of acrylamide at significantly higherdoses than are typically foundin food (Başaran et al., 2023).Various supplements such as: fennel, nigella, pomegranate, wild berries, cumin, black cumin, bamboo leaves and many other supplements have an effect on reducing acrylamide levels, which comes from their antioxidant activity (Al-Ansi et al., 2019; Ashkezari & Salehifar, 2019; Borczak et al., 2022; Abdel-Shafi Abdel-Samie et al., 2011; Li et al., 2012). These supplements, in additionto having an impact on the level of acrylamide, they also have antioxidant activity, antimicrobial activity, provide immune support and other health benefitsthat make productswith added ingredients like these functional products.
Keywords: acrylamide, additives, antioxidant activity, functional product
Introduction
Acrylamide is formed in heated food by the condensation of the amino group of an amino acid (asparagine) with the carbonyl group of a sugar. Asparagine needs a carbonyl compound to convert to acrylamide. The carbonyl can come from a number of sources. Due to the diversity of antioxidants in structure and properties and the complexity of reactions, differentantioxidants are involvedin different reactions during the Maillard reaction process, thereby causing different effects on the formation of acrylamide (Jin et al., 2013).
Antioxidants can inhibit acrylamide formation in high-temperature processed foods in three ways. The first is to destroy the formed acrylamide from their oxidized products.The second is the formation of quinones or carbonyl compounds such as vitamin C which then react with the main precursor of acrylamide andasparagine. The inhibition effect depends on how easily they are oxidized and the rate of oxidation and their oxidized products react with asparagine. The third is the inhibition of the production of carbonyl compoundsproduced by fryingoil (Ou et al., 2010).
Acrylamide inhibition studies include those by Cheng et al. (2015) who found that severalantioxidant flavones and isoflavones (apigenin, luteolin, naringenin, tricin,daicein, daicin, genistein, genistin) inhibited acrylamide formation by up to 52.1% in a model system, possiblyby reacting with key Maillardintermediates such as which is 3-aminopropionamide. The rate of inhibition was correlated with the change in the antioxidant capacity of trolox equivalent of the flavonoids measured by the DPPH assay. Depending on the concentration, naturalantioxidants can either favor or prevent acrylamide formation in model systems.
1Natural extracts of plant origin could come up with alternatives to synthetic preservatives, especially antioxidants, which also provide bioactive properties and some other benefits to the final products (Caleja et al., 2017). Several studies have reported that natural extracts of aromatic plants, spices and fruit extracts are used asantioxidants in meat, dairy and bakeryproducts (Gandhi et al., 2001;Caleja et al., 2017; Reddy et al., 2005). However, increasing interesthas been given to studiesof additives from natural sourcesas potential antioxidants. In recent years, a number of antioxidants of plant origin, mainly from medicinal and aromatic plants, have been shown to be valuable in delaying the process of lipid peroxidation in food products(Bajaj et al., 2006).
Polyphenols are recognized as secondary metabolites in fruits and vegetables, which have some functionalities including antioxidant, antitumor and anti-inflammatory activities. They are also able to modulate the immune system and protect the cardiovascular system (Munin & Edwards-Lévy, 2011). Several studies have shown that plant extracts containing polyphenols can reduce acrylamide content and increase antioxidant activity in many food systems (Fernández et al., 2003; Zhanget al., 2007; Marková et al., 2012).
Materials And Methods
Fennel (Foeniculum vulgare L.) belongsto the Apiaceae family, with a long historyof herbal applications and is widelycultivated in Egypt and India and is characterized by its edible leaves and strongly flavored seeds (Kulisic et al., 2004). The antioxidant activity of FS was analyzed by various antioxidant methods, including ABTS and DPPH. These antioxidant properties were contrasted with several standard syntheticantioxidants such as α-tocopherol, BHA (Butylated Hydroxyanisole) and BHT (Butylated Hydroxytoluene). FS ethanol and aqueous extracts showed strong antioxidant activity (Roby et al., 2013).
Black cumin seeds (Nigella sativa L.) commonly known as Nigella or black seed (BS), belonging to the familyRanunculaceae, is a well- known herb planted in Pakistan, India and Iran (Oktay et al., 2003). BS is enriched with nutritional benefits (Saxena et al., 2017). BS oil contains flavonoids, phenolic and some other related compounds, which increasedits antioxidant properties (Oktay et al., 2003; Dubeyet al., 2016).
Pomegranate flower extract (PFE) contains polyphenols (gallic and ellagic acids) and triterpenoids that have potentialantioxidant capabilities (Sreekumar et al., 2014). In addition, an inhibitory effectof water-soluble vitaminson acrylamide formationfrom fried potatoes has been reported; Water-soluble vitamins such as B2, B5 and B12 can reduce acrylamide synthesis by 20%, due to their strongantioxidant properties and structural stability. It is precisely these vitaminsthat are more effective in reducing the formation of acrylamide duringfood processing (Zenget al., 2009).
The nutrients of wild plants are comparable, sometimes even better than those of cultivated varieties (Sidor & Gramza-Michałowska, 2019; Borowska & Brzóska, 2016; Samec & Piljac-Zegarac, 2011; Zhang et al., 2001; Baltacioglu et al., 2011; Hukkanen et al., 2006; Polumackanycz et al., 2020; Lipowski et al., 2009; Kruczek et al., 2012; Sidor & Gramza-Michałowska, 2015; Młynarczyk et al., 2018;Cristea et al., 2021; Nazhand et al., 2020; Singh et al., 2021; Liu et al., 2022; Tereshchuk et al., 2020).Wild fruits that are cultivated are berry crops (bushes) classified in different families:Rosaceae (Aronia melanocarpa, Crataegus L., Sorbus aucuparia L., Rosa canina L.), Elaeagnaceae (Hippophae rhamnoides L.), and Adoxaceae(Sambucus nigra L.) (Sidor & Gramza-Michałowska,2019; Borowska & Brzóska, 2016; Samec & Piljac-Zegarac, 2011; Zhang et al., 2001; Baltacioglu et al., 2011; Hukkanen et al., 2006; Polumackanycz et al., 2020; Lipowski et al., 2009; Kruczek et al., 2012; Sidor & Gramza-Michałowska, 2015; Młynarczyk et al., 2018).
Black cumin (Nigella sativa L.) is unique in its nutritional profile, and black cumin fixed and essential oils have phytochemical-rich fractions(Tauseef et al., 2009).
Black cumin seeds possess high antioxidant activity (Mariod et al., 2009; Salih et al., 2009). Black cumin seeds are used to produce cakes with high TPC content, high DPPH without radicalscavenging abilities and a strong effect on reducing β-carotene oxidation. The dominant phenolic compoundsin black cumin cakes are hydroxybenzoic and β-coumaric acids (Mariod et al., 2009).
In a study by Abdel-Shafi Abdel-Samie et al. (2011),the antioxidant activity and acrylamide contentof biscuits with low-gluten flour and with the addition of cumin and black cumin in different amounts of 5%, 10%, 15% and lentil flourat a concentration of 0-15 g/ 100 g of flour.
Al-Ansi et al. (2019) made biscuits with added fennel and cumin seeds which were ground and added in differentamounts, the biscuitswere made in two batches:
- The first batch was baked in conventional conditions at 190 °C for 10 min,
- The second batch in the microwave at 700 W for 90 seconds.
These biscuits were analyzed for antioxidant activity and acrylamide levels which are presented in graphs 1 and 2.
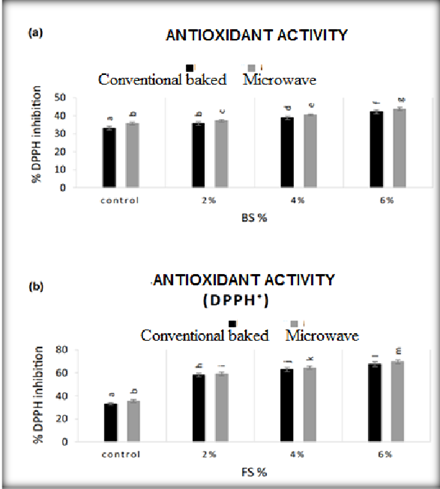
Graph 1. Antioxidant activity of biscuits with fennel and cumin seeds (BS% - cumin, FS% - fennel)
Antioxidant analyzes of FS in DPPH showed an IC50 value of 746.9 mcg/mL (Mallick et al., 2016). Conventional baked biscuits showed the lowest antioxidant activity and capacity and increased with increasing amountof BS or FS. However,the presence of natural additives provided some additional benefits.FS biscuits had higher antioxidant capacity and activity compared to BS biscuits. Similarresults were observed by Caleja et al. (2017) in their comparative study between natural and synthetic antioxidants. They reported that the addition of FS extract(as a natural antioxidant) provided similar antioxidant activity in biscuits compared to synthetic ones.
Liu (2007) reported that whole grains are rich in antioxidant phytochemicals. Antioxidant activity of FS extractsin ethanol and water shows strong antioxidant activity (Oktay et al., 2003). Higherantioxidant activity in microwave oven, for two samples of BS and FS biscuits was due to the ability of microwave oven, as stated by Mahdi et al. (2019) who observedthat the microwavetechnique has great significance in improving the release and extraction of bioactive compounds from plant sources

Graph 2. Level of acrylamides in biscuits with fennel and cumin (BS% - cumin, FS% - fennel)
Acrylamide content ranged from LOMQ to 355.2 μg/kg (Al-Ansi et al., 2019).According to the authors, the levels of acrylamide contentwere lower than what was indicated by the European Commission (500 mg/kg). The highest levels of acrylamide were observed in the control conventionally baked biscuits. Additionof BS reduced acrylamide formation to 17%, 31%, and 53% in conventionally baked biscuits, while reductions of 23%, 58%, and 68% were in microwave-baked biscuits to 2%, 4%, and 6% at BS (chart.2a). The authors also determined that the addition of FS significantly reduced acrylamide formation up to LOMQ in microwave-baked biscuitsand up to 78% in conventionally baked biscuits (graph. 2b).
- Effect of Pomegranate Flower Extract (PFE) and Vitamin B3 on Acrylamide Level and Antioxidant Activity in Donuts
Table 1 presents the level of acrylamide in donuts with added pomegranate flower extract and Vitamin B3, which were dried at room temperature and then ground into powder form (Ashkezari & Salehifar, 2019).
Table 1. Acrylamide content in donuts with pomegranate flower extractand Vitamin B3
| Resources | Acrylamide content µg/kg |
| Model(p) | <0> |
| A | <0> |
| B | 0,007 |
| AB | 0,5817 |
| A2 | 0,0042 |
| B2 | 0,3083 |
| R2 | 0,9601 |
| adapted R2 | 0,9316 |
The authors (Ashkezari & Salehifar, 2019) used the F model with a value of 33.68 and p value less than 0.0001 which indicated that the model was significant. In addition, the p values of the linear coefficients (A for PFE and B for vitamin B3) and the squared coefficient of determination (A2 for PFE) were less than 0.05. The coefficient of determination (R2) and the adjusted coefficient of determination (adjusted R2) were 0.96 and 0.93, which indicatedthat the model was suitablefor predicting the experimental data of acrylamide content in doughnuts.
The authorsfurther determined that the addition of PFE and vitaminB3 caused a decrease in the acrylamide content of donuts, mainly due to the antioxidant activities of the compounds, scavenging of carbonyls and limitation (scavenging) of the degradation of sugars
during the Maillard reaction(Urbančič et al., 2014). Ciesarová et al. (2008) reporteda similar result and showed that the addition of plant extracts derived from pimento,black pepper, marjoramand oregano to potatoes reducedthe acrylamide contentby up to 75%. Additionally, Budryn et al. (2013) reported that high concentrations of greentea and green coffee extractsreduced acrylamide formationin fried doughnuts.
1.Effect of wild fruits on acrylamide level and antioxidant activity in biscuits
Borczak et al. (2022),analyzed the level of acrylamides and antioxidant activity in wheat flour biscuits added (5% percent) six different wild fruits, namely: chokeberry, rowanberry, hawthrons (hawthorn), wild rose, elder and sea buckthorn berries( Sea buckthorn berries), which are presentedin table 2.
Table 2. Antioxidant activity and acrylamide contentin biscuits with added wild fruits
Chokeberry |
Wild Rose (Rosacanina L.) |
Bossel (Sambucus nigraL.) |
Hawthorn (Crataegus L.) |
Rowanberry (Sorbus aucuparia L.) | Sea buckthorn berries (Hippophae rhamnoides L.) |
Control | |
| Antioxidant activity | |||||||
ABTS µmol·g-1 во с.м |
15.22 ± 0.05 a |
5.38 ± 0.04 d |
9.42 ± 0.01 b |
7.58 ± 0.01 c |
7.61 ± 0.05 c |
4.99 ± 0.03 e |
1.11 ± 0.00 f |
FRAP µmol·g-1 во с.м |
17.47 ± 0.05 b |
26.12 ± 0.83 a |
11.37 ± 0.17 e |
12.30 ± 0.17 d |
13.66 ± 0.16 c |
9.83 ± 0.27 f |
2.46 ± 0.08 g |
| Acrylamide content | |||||||
Acrylamide µg·1000 g-1 вос.м |
81.98 ± 0.95 a |
173.90 ± 0.54 d |
120.26 ± 1.09 b |
524.96 ± 1.98 f |
370.63 ± 1.76 e |
136.06 ± 0.65 c |
1290.77 ± 1.23 g |
Regarding the acrylamide content, the authors determined that all additives showed a strong inhibition efficiency of the formation of acrylamide in the biscuit samples. The acrylamide content in the controlbiscuit sample was 361.2 μg/kg.The single additionof lentils to the biscuit formula reduced the amount of acrylamide formed to 346.8, 330.4 μg/kg and 288.3 μg/kg when lentils were added at 5% (T2), 10% (T3) and 15 % (T4). The addition of 15% lentils to the biscuit formulainhibited 20.2% of acrylamide formation in the biscuit controlsamples.
Cumin also reducedacrylamide formation to 285.2, 199.1 μg/kg and
117.0 μg/kg when cumin was added at 5% (T5), 10% (T9) and 15% (T13). The maximum addition of cumin (15% in T13) inhibited 67.6% of the acrylamide formed in the control biscuit samples. The combination of 15% lentils with 15% cumin (T16) reduced the acrylamide level to 66.7 μg/kg, (81.5% inhibition of acrylamide formation) compared to the biscuitcontrol samples.
Black cumin achieved the highest acrylamide inhibition among the additives. The addition of 5%, 10% and 15% black cumin to the biscuit formula reducedthe acrylamide contentof the biscuits to only
140.6, 104.7 μg/kg and 87.2 μg/kg. The addition of 15% black cumin to the biscuitformula reduced acrylamide formation by 75.9% comparedto control biscuitsamples. The combination of 15% buckwheat with 15% black cumin (T28) reduced acrylamide by 89.5% compared to control biscuit samples, resulting in the lowest acrylamide content of 38.0 μg/kg
Conclusion
The use of antioxidant compounds was found to be effective in reducing acrylamide formation due to three effects: (1) carbonyl trapping, (2) reductionof sugar degradation through Maillard reactionprocesses, and (3) radical scavenging activities. The relationship between acrylamide reductionand amounts of FS(fennel) or BS(cumin) was clearly positive,indicating that antioxidant levels increased with increasing amountsof BS or FS.
From the results of the study(Ashkezari & Salehifar, 2019) it can be concluded that the addition of pomegranate flower extract and vitamin B3 can have a positive effect on the nutritional value and reduction of acrylamide in doughnuts.
Due to its polyphenolic compounds, pomegranate flower extract increases the antioxidant property of the doughnut. The high antioxidant activity and structural stability of vitamin B3 leads to a significant reduction of the acrylamide content in the final product. In general, pomegranate flower extract and vitamin B3 can be used as food antioxidants and an alternative to synthetic preservatives to reduce the acrylamide contentof high-temperature-treated food products.
The addition of wild fruits can be an innovative approach in the production of functional biscuitswith improved antioxidant properties characterized by reduced levels of acrylamide, while maintaining organoleptic properties acceptable and desirable to potential consumers.
Increasing the level of addition of lentil flour,cumin and black cumin reducedthe acrylamide contentof prepared biscuits,despite increased acrylamide precursors in formulated flours. This may bedue to the high antioxidant activity of the flours formulated with the addition of lentil flour, cumin and black cumin.
References
-
Abdel-Shafi Abdel-Samie M., Wei-Ning H., Zhen-Ni M., Yuan Y. & Kim Chung O. (2011). Acrylamide Inhibition in Cookies Using Natural Antioxidants. Food Science, 32(7), 129-140.
Publisher | Google Scholor -
Al-Ansi W., Mahdi A., Al-Maqtari Q., Fan M. W., Li Y., Qian H. & Zhang H. (2019). Evaluating the role of microwave- baking and fennel (Foeniculum vulgare L.)/nigella (Nigella sativa L.) on acrylamide growth and antioxidants potential in biscuits. Journal of Food Measurement and Characterization volume, 13, 2426–2437.
Publisher | Google Scholor -
Ashkezari M. & Salehifar M. (2019). Inhibitory effects of pomegranate flower extract and vitamin B3 on the formation of acrylamide during the donut making process. Journal of Food Measurement and Characterization. Journal of Food Measurement and Characterization, 13(1), 735-744.
Publisher | Google Scholor -
Baltacioglu C., Velioglu S. & Karacabey E. (2011). Changes in total phenolic and flavonoid contents of rowanberry fruit during postharvest storage. J. Food Qual., 34, 278-283.
Publisher | Google Scholor -
Başaran B., Çuvalcı B. & Kaban G. (2023). Dietary Acrylamide Exposure and Cancer Risk: A Systematic Approach to Human Epidemiological Studies. Foods, 12(2), 346.
Publisher | Google Scholor -
Borczak B., Sikora M., Kapusta-Duch J., Fołta M., Szewczyk A., Zięć G. & Leszczyńska T. (2022). Antioxidative Properties and Acrylamide Content of Functional Wheat-Flour Cookies Enriched with Wild-Grown Fruits. Molecules, 27(17), 5531.
Publisher | Google Scholor -
Borczak, B., Sikora E., Sikora M., Kapusta-Duch J., Kutyła- Kupidura E. & Fołta M. (2016). Nutritional properties of wholemeal wheat-flour bread with an addition of selected wild grown fruits. Starch–Stärke, 68, 1-8.
Publisher | Google Scholor -
Borowska S., & Brzóska M. (2016). Chokeberries (Aronia melanocarpa) and their products as a possible means for the prevention and treatment of noncommunicable diseases and unfavorable health effects due to exposure to xenobiotics. Compr. Rev. Food Sci. Food, 15, 982-1017.
Publisher | Google Scholor -
Budryn G., Żyżelewicz D., Nebesny E., Oracz J. & Krysiak W. (2013). Influence of addition of green tea and green coffee extracts on the properties of fine yeast pastry fried products. Food Res. Int., 50, 149-160.
Publisher | Google Scholor -
Caleja C., L. B., A.L. A., M.B.P. O. & I.C. F. (2017). A comparative study between natural and synthetic antioxidants. Evaluation of their performance after incorporation into biscuits. Food Chem., 216, 342-346.
Publisher | Google Scholor -
Cheng J., Chen X., Zhao S. & Y. Zhang. (2015). Antioxidant capacity-based models for the prediction of acrylamide reduction by flavonoids, Food Chem. 168, 90–99.
Publisher | Google Scholor -
Ciesarová Z., Suhaj M. & Horváthová, J. (2008). Correlation between acrylamide contents and antioxidant capacities of spice extracts in a model potato matrix. J. Food Nutr. Res., 47, 1-5.
Publisher | Google Scholor -
Cristea E., Ghendov-Mosanu A., Patras A., Socaciu C., Pintea A., Tudor C. & Sturza R. (2021). The influence of temperature, storage conditions, pH, and ionic strength on the antioxidant activity and color parameters of rowan berry extracts. Molecules, 26, 3786.
Publisher | Google Scholor -
Dubey P., Singh B., Mishra B., Kant K. & Solanki R. (2016). Nigella (Nigella sativa L.): a high value seed spice with immense medicinal. Indian J. Agric. Sci, 86, 967-979.
Publisher | Google Scholor -
Fernández S., Kurppa L., & Hyvönen L. (2003). Content of acrylamide decreased inpotato chips with addition of a proprietary flavonoid spice mix (Flavomare®) infrying. Innov. Food Technol., 18, 24-26.
--> -
Gandhi A., Kotwaliwale N., Kawalkar J., Srivastav D., Parihar V. & Nadh P. (2001). Effect of incorporation of defatted soyflour on the quality of sweet biscuits. J. Food Sci. Technol, 38, 502-503.
Publisher | Google Scholor -
Hukkanen A., Pölönen S., Kärenlampi S. & Kokko H. (2006). Antioxidant capacity and phenolic content of sweet rowanberries. J. Agric. Food Chem., 54, 112-119.
Publisher | Google Scholor -
Jabłonska-Rys E., Zalewska-Korona M. & Kalbarczyk J. (2009). Antioxidant capacity, ascorbic acid and phenolics content in wild edible fruits. J. Fruit Ornam Plant Res., 17, 115- 120.
Publisher | Google Scholor -
Jin C., Wu X. & Zhang Y. (2013). Relationship between antioxidants and acrylamide formation: A review. Food Research International, 51(2), 611-620.
Publisher | Google Scholor -
Kruczek M., Swiderski A., Mech-Nowak A. & Król K. (2012). Antioxidant capacity of crude extracts containing carotenoids from the berries of various cultivars of sea buckthorn (Hippophae rhamnoides L.). Acta Biochim. Polon., 59, 135- 137.
Publisher | Google Scholor -
Kulisic T., Radonic A., Katalinic V. & Milos M. (2004). Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem., 85, 633-640.
Publisher | Google Scholor -
Li D., Chen Y., Zhang Y., Lu B., Jin C., Wu X. & Zhang Y. (2012). Study on mitigation of acrylamide formation in cookies by 5 antioxidants. Journal of food science, 77(11), C1144– C1149.
Publisher | Google Scholor -
Lipowski J., Marszałek K. & Skapska S. (2009). Sea Buckthorn-an innovative raw material for the fruit and vegetable processing industry. J. Fruit Ornam. Plant Res., 17, 121-126.
Publisher | Google Scholor -
Liu D., He X., Wu D., Li H., Feng Y., Zou L. & Gan R. (2022). Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J. Agric. Food Chem., 70, 4202- 4220.
Publisher | Google Scholor -
Liu R. (2007). Whole grain phytochemicals and health. J. Cereal Sci., 46, 207-219.
Publisher | Google Scholor -
Mahdi A. A., Rashed M. M., Al-Ansi W., Ahmed M. I., Obadi M., Jiang Q. & H. W. (2019). Enhancing bio-recovery of bioactive compounds extracted from Citrus medica L. Var. sarcodactylis: optimization performance of integrated of pulsed-ultrasonic/microwave technique. Food Measure , 13, 1661-1673.
Publisher | Google Scholor -
Mallick M., Bose A. & Mukhi S. (2016). Comparative evaluation of the antioxidant activity of some commonly used spices. Int. J. PharmTech., 9, 1-8.
--> -
Mariod A., Ibrahim R. & Ismail M. (2009). Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake[J]. Food Chemistry, 116, 306-312.
Publisher | Google Scholor -
Marková L., Ciesarová Z., Kukurová K., Zieliński H. & Przygodzka, M. (2012). Influence of various spices on acrylamide content in buckwheat ginger cakes. Chem Pap., 66, 949-954.
Publisher | Google Scholor -
Martinez E., Rodríguez J., Mondragon A., Lorenzo J. & Santos, E. (2019). Influence of Potato Crisps Processing Parameters on Acrylamide Formation and Bioaccesibility. Molecules., 24, 3827.
Publisher | Google Scholor -
Młynarczyk K., Walkowiak-Tomczak D. & Łysiak G. (2018). Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods, 40, 377-390.
Publisher | Google Scholor -
Munin A. & Edwards-Lévy F. (2011). Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics, 3, 793-829.
Publisher | Google Scholor -
Nazhand A., Lucarini M., Durazzo A., Zaccardelli M., Cristarella S., Souto S. & Santini A. (2020). Hawthorn (Crataegus spp.): An updated overview on its beneficial properties. Forests, 11, 564.
Publisher | Google Scholor -
Nematollahi A., Kamankesh M., Hosseini H., Ghasemi J., Hosseini-Esfahani F., Mohammadi A. & Mousavi Khaneghah A. (2020). Acrylamide content of collected food products from Tehran’s market: A risk assessment study. Environ. Sci. Pollut. Res., 27, 30558–30570.
Publisher | Google Scholor -
Oktay M., Gülçin İ. & Küfrevioğlu Ö. (2003). Determination of in vitro antioxidant activity of fennel (Foeniculum vulgare) seed extracts. LWT Food Sci. Technol, 36, 263-271.
Publisher | Google Scholor -
Olszowy M. (2019). What is responsible for antioxidant properties of polyphenolic compounds from plants? Plant Physiol. Biochem., 144, 135-143.
Publisher | Google Scholor -
Ou S., J. Shi, C. Huang, G. Zhang, J. Teng, Y. Jiang & B. Yang, (2010). Effect of antioxidants on elimination and formation of acrylamide in model reaction systems. J. Hazard. Mater., 182: 863-868.
Publisher | Google Scholor -
Polumackanycz M., Kaszuba M., Konopacka A., Marzec- Wróblewska U., Wesolowski M., Waleron, K. & Viapiana, A. (2020). Phenolic composition and biological properties of wild and commercial dog rose fruits and leaves. Molecules, 25, 5272.
Publisher | Google Scholor -
Reddy V., A. U. & Kumar A. (2005). Evaluation of antioxidant activity of some plant extracts and their application in biscuits. Food Chem., 90, 317-321.
Publisher | Google Scholor -
Roby M., Sarhan M., Selim K. & Khalel K. (2013). Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L) and chamomile (Matricaria chamomilla L). Ind. Crops Prod., 44, 437-445.
Publisher | Google Scholor -
Russo D. (2018). Flavonoids and the structure-antioxidant activity relationship. J. Pharmacogn. Nat. Prod., 4, 1.
Publisher | Google Scholor -
Salih B., Sipahi T. & Donmez E. (2009). Ancient nigella seeds from BoyalI Hoyok in north-central Turkey[J]. Journal of Ethnopharmacology, 124, 416-420.
Publisher | Google Scholor -
Samec D. & Piljac-Zegarac J. (2011). Postharvest stability of antioxidant compounds in hawthorn and cornelian cherries at room and refrigerator temperatures-comparison with blackberries, white and red grapes. Sci. Hortic., 131, 15-21.
Publisher | Google Scholor -
Saxena S., Rathore S., Diwakar Y., Kakani R., Dubey, P. & John S. (2017). Genetic diversity in fatty acid composition and antioxidant capacity of Nigella sativa L. genotypes. LWT Food Sci. Technol., 78, 198-207.
Publisher | Google Scholor -
Shivani Bajaj., Asna Urooj. & P. Prabhasankar. (2006). Effect of incorporation of mint on texture, colour and sensory parameters of biscuits. Int. J. Food Prop. 9691-700
Publisher | Google Scholor -
Sidor A. & Gramza-Michałowska A. (2019). Black chokeberry Aronia melanocarpa L.-A qualitative composition, phenolic profile and antioxidant potential. Molecules, 24, 3710.
Publisher | Google Scholor -
Sidor A., & Gramza-Michałowska A. (2015). Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food-a review. J. Funct. Foods, 18, 941-958.
--> -
Singh K., Singh D., Lone J., Bhat S., Sharma Y. & Gairola S. (2021). Nutraceutical potential of rose hips of three wild Rosa species fromWestern Himalaya, India. Not. Bot. Horti Agrobot. Cluj Napoca, 49, 12471.
Publisher | Google Scholor -
SNFA S. N. (2020, March). Acrylamide Is Formed during the Preparation of Food and Occurs in Many Foodstuffs. 10. Повратено од http://www.slv.se/engdefault.asp
--> -
Sreeja Sreekumar., Hima Sithul., Parvathy Muraleedharan., Juberiya Mohammed Azeez. & Sreeja Sreeharshan. (2014). Pomegranate fruit as a rich source of biologically active compounds. Biomed. Res. Int.1-12
Publisher | Google Scholor -
Tauseef S. M., Butt M. S. & Anjum, F. M. (2009). Safety assessment of black cumin fixed and essential oil in normal Sprague Dawley rats: serological and hematological indices. Food and Chemistry Toxicology, 47, 2768-2775.
Publisher | Google Scholor -
Tereshchuk L., Starovoytova K., Babich O., Dyshlyuk L., Sergeeva I., Pavsky V. & Prosekov, A. (2020). Sea buckthorn and rosehip oils with chokeberry extract to prevent hypercholesterolemia in mice caused by a high-fat diet in vivo. Nutrients, 12, 2941.
Publisher | Google Scholor -
Urbančič S., Kolar M., Dimitrijević D., Demšar L. & Vidrih R. (2014). Stabilisation of sunflower oil and reduction of acrylamide formation of potato with rosemary extract during deep-fat frying. LWT. Food Sci. Technol., 57, 671- 678.
Publisher | Google Scholor -
Viuda-Martos M., Fernández-López J. & Pérez-Álvarez J. (2010). Pomegranate and its many functional components as related to human. Compr. Rev. Food Sci. Food Saf., 9, 635-654.
Publisher | Google Scholor -
WHO. (2005). Summary Report of the Sixty-Fourth Meeting of the Joint FAO/WHO Expert Committee on Food Additive (JECFA). Washington, DC, USA: The ILSI Press International Life Sciences Institute., 1-47, ISBN 9241209305.
--> -
Xiaohui Zeng., Ka-Wing Cheng., Yue Jiang., Zhi-Xiu Lin., Jian-Jun Shi., Shi-Yi Ou., Feng Chen. & Mingfu Wang. (2009). Inhibition of acrylamide formation by vitamins in model reactions and fried potato strips. Food Chem. 11634- 39
Publisher | Google Scholor -
Zhang Y., Xu W., Wu X., Zhang X. & Zhang Y. (2007). Addition of antioxidant from bamboo leaves as an effective way to reduce the formation of acrylamide in fried chicken wings. Food Addit. Contam., 24, 242-251.
Publisher | Google Scholor -
Zhang Z., Chang Q., Zhu M., Huang Y., Ho W. & Chen Z.- Y. (2001). Characterization of antioxidants present in hawthorn fruits. J. Nutr. Biochem., 12, 144-152.
Publisher | Google Scholor

