RESEARCH ARTICLE
1independent researcher applied pharmacologist , Hospital pharmacist manager , italy PC area 29121
2Professor, Physiology, College of Medicine, University of Babylon, Hilla, Iraq
3Professor of physical chemistry, Libyan Authority for Scientific Research
4Mashori G.R. Professor, Department of Medical & Health Sciences for Woman, Peoples University of Medical and Health Sciences for Women, Pakistan
5medical laboratory Turin , Citta della salute -italy
6 IMA academy President
*Corresponding Author: Luisetto M, independent researcher applied pharmacologist, Hospital pharmacist manager, italy PC area 29121
Citation: Luisetto M 1*, ACIDO LABILE OR GASTRO IRRITANT APIs AND ENTERIC RELEASE IN GALENIC PRACTICE: AN OVERVIEW, Digestive System and Hepatobiliary Diseases, vol 1(1). DOI: https://doi.org/10.64347/3066-3040/DSHD.005
Copyright: © 2024, Luisetto M 1*, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: November 07, 2024 | Accepted: November 10, 2024 | Published: November 28, 2024
Abstract
Aim of this work is to verify the acid lable , gastro irritants APIs for oral subministration and the active molecule that need a specific enteric release.
This information are useful in galenic field in order to choose the best veicle (for oral suspension or
solution ) and the right kind of capsules ( normal or with gastroresistance ).
In this article not all molecules are analyzed , only submitted significative example.
Some formulation are reported that require specific PH or enteric release or based on lightly water soluble API.
Various charcteristics of some commercial product ( veicle for oral suspension) are reported only to show the composition and the rationale of their use : the reason to use one veicle for ph 4 or for buffered at ph 7-8 or and related the capsules : use of normal or AR or treated for gastroresistence - enteric coating.
The chemico-phisical properties of the active principle are crucial to choose the right veicle for oral suspension or the kind of capsules ( gastroresistence or not ) .
Fundamental for this approach the physiology of the Ph variation along the GI apparatus .
Keywords: chemico-phisical properties , physiology, acid -labile API, gastro irritants API, enteric release, galenic, gastroresistance, Ready for use veicle for oral suspension , gastro resistence cps, solubility, compatibility , stability
Introduction
In the galenic practice is needed to verify before to start the preparation to verify the compatibility of the APIs with the excipients : for oral solution and suspension the solubility and the stability in acid-basic environment and the need or not for a controlled release in the GI tract.
it is crucial to observe the PH variation along the GI tract for the implication in the galenic practice : there is a great
variation from ph 1,5-4 in the stomach since ph 7 in small and large intestine and 8,5 in Duodenum
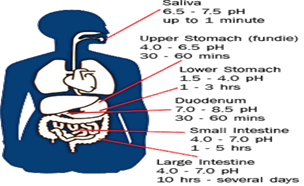
Fig n 1 GI PH variation
Related the specific pharmaceutical form needed and its profile of release various literature are of interest :
S.T.P. PHARMA SCIENCES 1999
Formulation and stability evaluation of enteric-coated omeprazole formulations
S. Bozdag, S. Çalis and M. Sumnu
“The most important reasons for enteric coating can be summarized as follows:
- to protect acid-labile drugs from the gastric fluid (like enzymes
and certain antibiotics),
- to prevent the distress or nausea due to irritation from a drug (like sodium salicylate),
- to deliver the drugs intended for local action in the intestines (intestinal antiseptics could be delivered to their site of action in a concentrated form and bypass systemic absorption in the stomach)
- to deliver the drugs that are optimally absorbed in the small intestine to their primary absorption site in their most concentrated form,- to provide a delayed-release component for repeat action tablets .”
From Khulbe et al., IJPSR, 2017; Vol. 8
“Acid labile drug”is a drug that is easily destroyed in acidic environment.
Stomach is the main site for drug absorption mainly by the oral rout. The pH of the stomach is acidic so the
absorption of acid labile ( AL) drugs through stomach is difficult.
The most commonly used acid labile AL drugs are amylase, aureomycin, bacitracin, beta carotene,
cephalosporins, Chloromycetin, cimetidine,cisapride, clorazepate, deramciclane,
didanosine, digitalis glycosides,dihydrostreptomycin, erythromycin, etoposide,famotidine, hormones ( estrogens, insulin, adrenalin ,heparin), lipase, ,novobiocin, pancreatin, penicillin salts, polymyxin, pravastatin, progabide, protease,quinapril, ranitidine, streptomycin, sulphanilamide or esomeprazole, lansoprazole, omeprazole, pantoprazole or rabeprazole. Amylase, lipase, protease , PPI are most commonly used drug which are unstable in acidic environment i.e. acid labile. PPI are used to supress the acid of the stomach. The use of Proton pump inhibitors is not limited it is also used for the suppression of ulcer related to stress and nonsteroidal anti-inflammatory drugs (NSAIDs).Long term use NSAIDs causes serious ulcer ”
REASONS FOR ENTERIC COATING :
To protect the stomach from the drug or To protect the drug from the stomach ,
To release the drug after the stomach : into the intestine tract
To protect the acid liable drugs from the gastric fluid ( enzymes and certain antibiotics)
To forbid gastric distress /nausea due to irritation from a drug ( sodium salicylate )
To deliver drugs intended for local action into the intestines tract, like intestinal antiseptics
could be delivered to their site of action in a concentrated form.
Need for minimizing 1th pass metabolism.
To extend a delayed release DR component for repeat- action tablets.”
Related the APIs and its characteristics it is possible to classify :
Between the ACIDO labile APIs :
Penicillin salts,Amoxicillin,Erythromycin ,proton pump inhibitors (or "PPIs") such as Lansoprazole, or Omeprazole; pancreatin,digitalis ,Furosemide, duloxetin, Budesonide, Vivotif
GASTRO IRRITANTS : Acid valproic ,Ferrum salts,Teofillin,Tiazide,anticoagulants, Fans like diclofenac , naproxen, anticoagulants ( Noacs) , strong electrolite like ammonium cloride, potassium cloride
(In example ferrograd RP is in commerce and KCL retard)
ENTERIC RELEASE IS NEEDED in example for : Budesonide ( modified release ), Tiamin ( absorbed in the small intestine : there are gastroresistence cp avaiable)
Related the solubility is relevant to observe that :
Volume 657 , 25 May International Journal of Pharmaceutics
Exploring paediatric oral suspension development: Challenges, requirements, and formulation advancements
Sachin S. Gaikwad , Javier O. Morales , Narayan B. Lande , Johanna Catalán-Figueroa
Umesh D. Laddha , Sanjay J. Kshirsagar
“The main reason for the development of an pharmaceutical suspensions PS is because of drug poor water solubility.”
Materials and Methods
With an observational approch some relevant literature is reported ( fro 1-to 11) and anlyzed related the topic under investigations. (Various figure from 1 to 9 help in the general meaning .)
Some formulation or veicle composition are also reported .
An experimental project hypotesys is submitted and finally a global conclusion is reported with a suggested rational way to follow in choosing the right excipients-veicle to be used in orals suspension or Capsules in the galenic practice.
Result
FORM LITERATURE
According Christine M Geiger et al
“The stability of 10 active pharmaceutical ingredients APIs was studied in SyrSpend SF PH4 or SyrSpend SF Alka at room and/or refrigerated temperature (2°C to 8°C). An oral suspension OS of each active pharmaceutical ingredient was compounded in low actinic plastic bottles at a specific concentration in SyrSpend SF PH4 or SyrSpend SF Alka.
Furosemide was stable for at least 14 days in SyrSpend SF Alka at refrigerated conditions. Prednisolone sodium phosphate in SyrSpend SF PH4 was stable for at least 30 days at room temperature RT and refrigerated conditions. Ranitidine hydrochloride suspensions in SyrSpend SF PH4 at room temperature RT and refrigerated conditions were stable for at least 30 days and 58 days, respectively. Hydrocortisone hemisuccinate and sodium phosphate retained greater than 90% for at least 60 days at both room temperature and refrigerated samples in SyrSpend SF PH4. Amiodarone hydrochloride and nifedipine suspensions at both room temperature RT and refrigerated conditions retained greater than 90% of the initial concentrations for at least 90 days in SyrSpend SF PH4. Refrigerated samples of simvastatin in SyrSpend SF PH4 were stable for at least 90 days. Spironolactone in SyrSpend SF PH4 at room temperature RT retained more than 90% of the initial concentration for at least 90 days. Phenobarbital PHB in SyrSpend SF PH4 retained above 90% of initial concentration for at least 154 days at room temperature. This studywork demonstrated the stability of a wide range of frequently used active pharmaceutical ingredients, tested in SyrSpend SF PH4 and SyrSpend SF Alka at different storage conditions.” (1)
Sarah O'Donnell et al
“Budesonide is a synthetic steroid of the glucocorticoid family with a high topical antiinflammatory activity. Enteric-coated EC formulations resist gastric-acid degradation, delivering active drug AD to the small intestine and proximal colon” (2)
Trang T et al
“Using enteric coating EC is useful to bypass the gastric pH barrier and prevent gastric inactivation of the pancreatic enzymes”(3)
From https://remedy.bnssg.icb.nhs.uk/media/6429/treatment-of-iron-deficiency-anaemia-in-adults-final.pdf
“Adverse effects of iron:include constipation, diarrhoea, epigastric pain, faecal impaction, gastrointestinal GI irritation and nausea.” (4)
From Uniphyllin Continus 400mg prolonged-release tablets technical sheet :
theophylline monohydrate
“between the undesiderable effetc: abdominal pain,Diarrhoea,Gastric irritation,Gastro-oesophageal reflux
Nausea / Vomiting”
Rabia Bushra et al
“Ibuprofen is a propionic acid derivative ( an NSAIDs). Major adverse reactions associated with Ibuprofen are related to GIT and include peptic and mucosal ulcers, dyspepsia, severe gastric pain , bleeding, that results in excessive treatment failure.”(5)
A Prakash et al
“Oral delayed-release mesalazine is an enteric-coated EC formulation which releases mesalazine in the terminal ileum and colon.”(6)
Dmitry S. Bordin et al
“It is assumed that anticoagulants may increase the risk of bleeding from the gastrointestinal GI tract through several mechanisms or their combinations:
(1) a systemic anticoagulant effect;
(2) local anticoagulant effect;
(3) local irritant effect; and
(4) a local action of the drug not that is not associated with coagulation (in example, the inhibition of mucosal healing) .”(7)

Fig n 3 From Fagron: Sysrpend ALKA composition
Kenneth T Moore et al
“Because some patients have difficulty swallowing a whole tablet, we investigated the relative bioavailability of a crushed 20 mg rivaroxaban tablet and of 2 alternative crushed tablet dosing strategies.
Stability and nasogastric NG tube adsorption characteristics of a crushed rivaroxaban tablet were assessed. In 55 healthy adults, relative bioavailability of rivaroxaban administered OS as a whole tablet , crushed tablet in applesauce suspension , or crushed tablet in water suspension via NG tube were determined.

Fig. n. 4 Formulation of some PPI oral suspension
There were no significant changes in mean % of non-degraded rivaroxaban recovered over 4 h from crushed tablet suspensions or after NG tube exposure . Relative bioavailability BA was similar between the Crushed-Oral and Reference dosing (Cmax and AUC∞ were within the 80-125% bioequivalence limits). Relative bioavailability RB was also similar between Crushed-NG and Reference dosing (AUC∞ was within bioequivalence limits; Cmax [90% CI range: 78.5-85.8%] was only slightly below the 80% lower bioequivalence limit).
A crushed rivaroxaban tablet was stable and when administered OS or via the NG tube, displayed similar relative bioavailability compared to a whole tablet administered orally.” (8)
Antonio Spennacchio et al
“Omeprazole is the progenitor of PPI . It is used for the treatment of ulcer and gastroesophageal GE reflux in dosages ranging from 10 mg/day to 40 mg/day, calibrated according to the patient's age and body weight. In this study work , the authors provide a report on the preparation of an extemporaneous liquid formulation of omeprazole using the fast oral solution Chopin a hydroxypropyl-?-cyclodextrin liquid base (pH 8 to 9) that is able to solubilize the drug. A solubility study of the drug in the liquid vehicle and a physical-chemical stability study of the 1-mg/mL formulation at 4°C and 25°C were performed. Analyses were carried out by using a HPCL method. Results showed that the intrinsic solubility IS of the drug in Chopin base was 5.33 mg/mL ± 0.23 mg/mL at 25°C and that omeprazole was chemically stable when the formulation was stored at 4°C over a period of 3 months, while its shelf life SL at 25°C was only 9 days. This work demonstrated that the resulting liquid formulation is suitable for all patients, in particular children or adults who are unable to take other pharmaceutical dosage PD forms, which overcomes the limitations of the medicines currently available on the market”. (9)
Daniel Banov, et al
“Oral vehicles play a critical role in the formulation of oral liquid medicines and are particularly important in addressing the needs of special patient populations . These vehicles are used to create solutions and suspensions of water-soluble and water-insoluble drugs, providing the desired stability, viscosity, pH and taste-masking capabilities.Unispend Anhydrous is a plant-based, anhydrous oral suspending AOS vehicle that is naturally sweetened. It contains medium-chain triglycerides TG , glyceryl distearate and polyglyceryl-3 oleate as its key ingredients. The vehicle includes a bitterness-masking agent to enhance its palatability. It is especially suitable for APIs that are unstable in water, are lipophilic or whit unknown aqueous stability” (10)
(Depakin 200 mg/ml 40 ml oral solution eccipient list : urea, NaOH, purified water as reported in the technical sheet )
Acid valproic oral suspension : it is used UNISPEND as veicle reltated the sligltly solubility in water of this API. (less than 1 mg/mL at 72 °F) and its lipofilic characteristic.
From US PHARMACIST Bosentan oral suspension 6,25 mg/ml
“Thoroughly pulverize the Tracleer (bosentan monohydrate) tablets (as 10 of the 62.5-mg or five of the 125-mg tablets) to a fine powder. Incorporate sufficient glycerin to form a smooth paste. Add sufficient Flavor Plus:Flavor Sweet (1:1) slowly with thorough mixing to final volume, and mix well. Package and label.

Fig. n. 4 Formulation of some PPI oral suspension
From SIFAP: italian Society of compounding pharmacist :

Fig. n 5

Fig n 6 from US PHARMACIST
From SIFAP procedure IBUPROFEN ORAL SUSPENSION:
“ the pharmacist can evaluate for the preparatio of the oral suspensio of Ibuprofenethe use of “ ready for use basis ” like : Base liquida per sospensione Acef; Base per sospensione orale Galeno; Fast Oral
Solution Wagner – Farmalabor SyrSpend SF PH4 Fagron; o altre analoghe; in this case assign hte dta of last use also according the indicatons or stability stydy provided by the producer and proceed with the controls on the final products as required .”

Fig n 7 from GPPG Oral Liquid Formulary V2 - Aug 2018.pdf
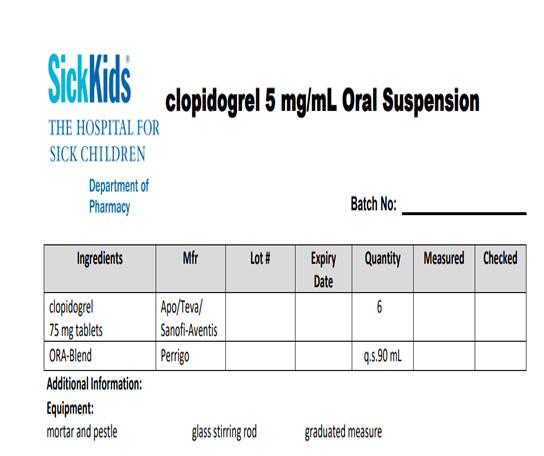
 Fig n 8 form Sickkids , Ora-Blend® is buffered to a slightly acidic pH (4.2) to help maintain preparation stability.
Fig n 8 form Sickkids , Ora-Blend® is buffered to a slightly acidic pH (4.2) to help maintain preparation stability.
Preeti Khulbe et al
“Buffer formulation: dosages forms which are used to increase the acid stability of the
drug by increasing the pH of the site of the release.
These formulations increase the pH of the delivery site and then release the drug so that it
does not appear in acidic environment AE . Buffers can classified as : water soluble and water insoluble buffers.
Water insolulbe buffer are: aluminum hydroxide, dihydroxy aluminum sodium carbonate,
calcium carbonate, aluminum phosphate, Al carbonate, Mg hydroxide dihydroxy
aluminum AL amino acetate, Mg oxide, magnesium trisilicate, aluminum phosphate,
magnesium carbonate and their combinations. Examples of water soluble buffer :
tripotassium phosphate, meglumine, sodium NA carbonate, sodium citrate, Ca gluconate,
disodium hydrogen phosphate, sodium bicarbonate dipotassium hydrogen phosphate, sodium tartarate,
sodium acetate, calcium CA glycerophosphate, tromethamine, magnesium oxide and their
combinations. It has been observed that in situ buffered formulation is an effective approach for
acid labile drugs ALD . There are many formulations which could enhance the acid stability of the drugs
but buffered formulation approach is the most effective and low cost approach. “. (11)
PRACTICAL EXPERIENCE
In orde to list the drugs that need GR capsules or veicle for oral suspenion for acido labile APIs it is
Suggeted to search in the official national formulary IT the drugs with AIC and pharmaceutical form:
Gastroresistence , modified release , enteric release delay relase.
Then it is needed to verify the list of the most common galenic formularion requested in pediatry or geriatry
Crucial to verify the composition of the registered drugs about the kind of excipients or gastroresistence coating ( or GR pellets inside normal hard capsules) .
Finally it is relevant to observe the composition of the various veicle for oral suspension in commerce :
related the ones for ph 4 and the other for PH 7-8, or for water instable APIs.
After all this it is possibile to list the APIs to be protected by the gastric acid environment or to release the active principle in small intestine or enteric release .
This list can be used in the local compounding laboratory .
Discussion
Currently in the there are various kind of veicle for oral suspension : generally this are designed or for APIs not gastro sensible with a PH about 4 or veicle buffered at ph 7- 8 for the active molecule instable in the gastric environment .
The same can be used hard capsules with gastro protection procedure ( acetoftalate in aceton bath or
eudragit ) when it is needed to protect from the gastric degradation the APIs or if it is needed to protect the gastric wall from gastro irritant principle .
Other specific condition need to delivery the drugs into the intestine environment ( like Budesonide in some inflamatory disease m. Chron’s) to provide an efficacy therapy.
For this reason in galenic practice it is crucial to verify before to choose the excipients or the kind of capsule if the molecule used for drugs preparation is gastro sensible , or gastro irritants or need a specific delay.
In this work are reported only some significative example that can be of interest for the pharmacist in every day work.
It is useful to verify the excipient and pharmaceutical form used in the registered drugs : in example if used gastroresitence capsules or enteric coated and the composition of ready for use veicle for oral suspension.
The same to observe the formulation officially in use as well as literature and other information about
Stability of the drugs or gastro irritants characteritics or acid -lability . ( see the technical sheet , literature and other publication).
Mandatory to follow the best practice, protocols and procedure adopted ( national or international ) and the pharmacopea requirements ( parameter for gastroresistence od capsules or quality control of oral suspension ).
Useful to verify in the official national prontuary the use of gastro resistence capslues or delay release or enteric in the drugs approved.
In the compounding activity ( reduction of doses for pediatry ) It is crucial to observe if the capsules of registered drugs for adults are only simply coated or gastro resitence coated or with GR pellets inside or delay release with enteric coating ( EUDRAGIT based or other polimer ).
Many times pharmaceutical industry ( for acido labile APIs or gastro irritants ) use normal gelatin capsules with inside pelletes coated with gastroresistence polimers. ( this pellets must not to be crashed to avoid the acid inactivation).
Other interesting source of information are the tecnical sheet of ready for use veicle for oral suspension as well as their compatibility table for the various APIs. ( ph 4 or ph > 7 )
To be considered also if the drug is to be taken in an full stomach or not. ( empty ph=2 , full ph about 4 )

 Acid-labile ( needed buffered veicle OS at Ph > 7)
Acid-labile ( needed buffered veicle OS at Ph > 7)
APIs gastro irritant ( needed gastro resistence system)
Enteric release needed ( needed controlled release)
(For instable in water APIs or high lipofilic consider a specific veicle )
Fig n 9 flow chart for excipient / cps to be used
Conclusion
The need to protect APIs from the acid environment of the stomach or to protect the gastric mucose from gastro irritant drugs ( or the need to release the active principle in the small intestine o in Other enteric district ) require to have deeply information about the chemico -phisical properties of the active molecule to be used .
If acido labile active principle it is mandatory to protect vs gastric acid or to delay the release in the intestine in order to increase efficacy .
In the galenic practice it is useful to cross the kind of of the registered drugs used for adults for preparing oral suspension or capsules ( reduction of doses for pediatry ) with the composition of the various ready for use veicle available .
Also in order to choose the kind of capsules to be used ( normal , Acido Resistance or GR coated ).
In an suggested flow chart it is necessary to verify two conditions :
- if the API is acid- labile (or not )in order to choose a veicle with a ph 4 or instead buffered at 7-8
- if the solubility of the API in water is low , lipofilic or it is instable in water . ( in this last situation to be evaluated a specific veicle).
It is relevant to verify also in the official national formulary what registered drugs for galenic interest are avaiable for an API if GR pharmaceutical form or delay release .
In every way the compatibility of the APIs with the excipients is crucial for ste stability of the final products, efficacy and safety. ( for newborn, pediatric or adults patients)
CONFLICT OF INTERESTS: NO
References
-
Int J Pharm Compd. 2015 Sep-Oct;19(5):420-7. Stability Assessment of 10 Active Pharmaceutical Ingredients Compounded in SyrSpend SF Christine M Geiger, Bridget Sorenson, Paul Whaley
--> -
Therapeutic benefits of budesonide in gastroenterology Sarah O'Donnell and Colm A. O'Morain https://doi.org/10.1177/20406223103792
Publisher | Google Scholor -
Trang T, Chan J, Graham DY. Pancreatic enzyme replacement therapy for pancreatic exocrine insufficiency in the 21st century. World J Gastroenterol 2014; 20(33): 11467-11485 DOI: 10.3748/wjg.v20.i33.11467
Publisher | Google Scholor -
https://remedy.bnssg.icb.nhs.uk/media/6429/treatment-of-iron-deficiency-anaemia-in-adults-final.pdf
--> -
Braz. J. Pharm. Sci. 46 (1) Mar 2010 https://doi.org/10.1590/S1984-82502010000100011 Enteric coating of ibuprofen tablets (200 mg) using an aqueous dispersion system Rabia Bushra, Muhammad Harris Shoaib, Nousheen Aslam, Zafar Alam Mehmood,Durriya Hashmat
--> -
Drugs . 1999 Mar;57(3):383-408. doi: 10.2165/00003495-199957030-00013. Oral delayed-release mesalazine: a review of its use in ulcerative colitis and Crohn's diseaseA Prakash , A Markham
--> -
Diagnostics (Basel). 2023 Jul; 13(13): 2220.doi: 10.3390/diagnostics13132220 Drug-Associated Gastropathy: Diagnostic Criteria Dmitry S. Bordin, Maria A. Livzan, Olga V. Gaus, Sergei I. Mozgovoi, and Angel Lanas Giuseppe Ingravallo
--> -
Randomized Controlled Trial Clin Pharmacol Drug Dev. 2014 Jul;3(4):321-7. doi: 10.1002/cpdd.123. Epub 2014 May 16.
--> -
Int J Pharm Compd . 2023 May-Jun;27(3):250-255.Stability of Omeprazole Extemporaneous Oral Solution in Chopin BaseAntonio Spennacchio , Angela Assunta Lopedota , Flavia Maria la Forgia , Sergio Fontana , Nunzio Denora , Antonio Lopalco
--> -
Pharmaceutics. 2023 Nov; 15(11): 2642.2023 Nov 20. doi: 10.3390/pharmaceutics15112642Analysis of the Physical Characteristics of an Anhydrous Vehicle for Compounded Pediatric Oral LiquidsDaniel Banov, Yi Liu, Kendice Ip, Ashley Shan, Christine Vu, Oleksandr Zdoryk, August S. Bassani, and Maria Carvalho,Arvind K. Bansal, Smita Salunke, and Daniel Schaufelberger
--> -
Khulbe et al., IJPSR, 2017; Vol. 8(1): 35-44. Received on 19 July, 2016; received in revised form, 13 September, 2016; accepted, 16 November, 2016; published 01 January, 2017 IN-SITU BUFFERED FORMULATION: AN EFFECTIVE APPROACH FOR ACID LABILE DRUG Preeti Khulbe , Birendra Shrivastava, Pankaj Sharma and Ajay Kumar Tiwari
--> -
Raccomandazione n.19 Ministero della salute IT Raccomandazione per la manipolazione forme farmaceutiche orali solide.
--> -
Valutazione della divisibilità e frantumabilità di forme farmaceutiche orali solide SIFO Manuale 2016
--> -
https://compoundingtoday.com/OralVehicle/ by the international journal of pharmaceutical compounding
--> -
Bettiol Manual for galenic preparation V edition Tecniche nuove editore
-->
