RESEARCH ARTICLE
- Kaniz Farhana Bithi 1
- F M Monjur Hasan 2
- Momen Khan 3
- Sumaiya Jahan 4
- Richmond Ronald Gomes 5
- Shoriful Islam 6
1. Junior Consultant, Medicine, National Institute of Neuroscience, Dhaka Bangladesh
2. Associate Professor, Ad din SakinaWomen’s Medical College,Jashore, Bangladesh
3. Medical Officer,Medicine, Dhaka MedicalCollege Hospital, Dhaka,Bangladesh
4. Graded Specialist, CMH, Dhaka, Bangladesh
*5. Professor, Medicine, Ad din Women’s MedicalCollege Hospital, Dhaka,Bangladesh
6. Senior Consultant, Medicine, 250 beddedGeneral Hospital, Jashore,Bangladesh
*Corresponding Author: Prof. Dr. Richmond Ronald Gomes*, Professor of Medicine,Ad-din Women’s Medical College Hospital2 Bara Maghbazar, Dhaka Bangladesh
Citation: Prof. Dr. Richmond Ronald Gomes*, (2024), Thyroid Dysfunction in patients with Nephrotic Syndrome, Global Journal of Clinical Nephrology (GJCN) 1(1), DOI:https://doi.org/10.64347/3066-2826/GJCN.003
Copyright: © ( 2024) Prof. Dr. Richmond Ronald Gomes*, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: April 23, 2024 | Accepted: May 24, 2024 | Published: May 28, 2024
Abstract
Background: Nephrotic syndrome is a nonspecific kidney disorder characterised by Proteinuria, hypoalbuminaemia, oedema & hyperlipidemia. In nephrotic syndrome there is marked increased in the glomerular permeability to macromolecules.
Aims: To asses thyroidstatus in patientwith nephrotic syndrome;To measure FT3, FT4 and TSH in patient with nephrotic syndrome and Level of proteinuria with Thyroid hormone level.
Methods: After fulfilling inclusion and exclusion criteria 50 diagnosed cases of idiopathic NS admitted in Nephrology unit of Rajshahi Medical College Hospital were included in this study. Thyroid status was evaluated in all the patients. In patients with NS mean (± SD) of FT4, FT3 and TSH were 39.34 (±29.49), 1.05 (±0.83) and 11.34 (±18.15) respectively. Significant positivecorrelation was found between serum albumin and serum FT4 (r = +0.733,p
<0.001) and also with total FT3 (r = +0.762,p <0.001). But no correlation was found betweenserum albumin and serum TSH (r = - 0.670, p<0.001) in patients with NS. So, it was found that nephrotic range proteinuria is associated with thyroid hormoneloss in urine and this can lead to significant reduction in serum FT4, FT3 and significant increase in serum TSH. Patients with NS can develop subclinical hypothyroidism or even overt hypothyroidism. Conclusion: Thyroid hormones are necessary for the growth and development of kidney. On the contrarydifferent form of kidney diseases are associated with various types of thyroid dysfunctions. This association between renal disease and thyroid dysfunction has been known for years.In patients with nephrotic syndrome(NS) large amount of protein is lost in urine along with thyroid hormones and hormone binding proteins. This hormone loss may lead to low FT4, FT3 and sometimes high TSH level
Keywords: Nephrotic syndrome, Proteinuria, thyroid dysfunction.
Introduction
The interactions between kidney and thyroid functions are known for years. Thyroid hormones are necessary for growth and development of kidney and for the maintenance of water and electrolyte homeostasis. On the other hand, decline in of kidney function is accompanied by changes in the synthesis, secretion, metabolism and elimination of thyroid hormone. Kidney disease such as glomerular or tubular disease, nephrotic syndrome, acute kidney injury, CKD and dialysis are known to causes different forms of thyroid dysfunction (Iglesias P & DIez JJ, 2009).
Nephrotic syndrome refers to a secondary phenomenon that occurs when substantial amount of protein are lost in the urine. It is characterized by overt proterinuria- usually >3.5 g/ 24 hours, hypoalbuminemia (< 30g>
annual incidence of nephrotic syndrome in children was found ranging from 2-7 per 100,000 and prevalence from 12-16 per 100,000 (Bagga A & Mantan M, 2005). A national population-based cross-sectional survey in Australia have shown the prevalence of proteinuria in adult is 2.4% (Steven J et al, 2003). Although a minimum annual incidence of nephrotic syndrome is 9.0 cases per million adult population (Sharpstone P, Ogg CS & Cameron JS, 1969), it is difficult to establish prevalence of nephrotic syndrome in adult because the condition is usually a result of an underlying disease.
In patient with nephrotic syndrome urinary losses of albumin are not fully compensated by the increased hepatic productions with hypoalbuminemia as a consequence. In patients with proteinuria many other proteins besides albumin are lost in the urine. Among these are hormones and hormone binding proteins (Gilles R et al, 2008). Several studies have documented urinary loss of thyroid hormones and thyroxin binding globulin (TBG) in patient with proteinuria (Feinstein EI et al. 1982, Iglesias Pet al. 2009, Junglee NA et al. 2006, KapteinEM et al. 1982 & Kaptein EM et al. 1991). Urinary losses of binding proteinssuch as thyroxinbinding globulin (TBG), thransthyrrectin or prealbumin, albumin and thyroid hormone bound to them result in a reduction in serum total thryroxin(T4) and sometimesin total T3 levels. These changes are related both to degree of proteinuria and to serum albumin levels (Feinstein EI et al, 1982 & Iglesias P et al, 2009). In patient with nephrotic syndrome losses of thyroid hormones may also lead to low free thyroid hormone levels unless production is increased under the influence of thyroid stimulating hormone (Gilles R et al, 2008). So nephrotic syndrome was known to be associated with changes in serum thyroid hormonelevels, ie- thyroxin(T4), T3 and TSHfor many years (Iglesias P et al, 2009, JungleeNA et al. 2006, Kaptein EM et al. 1982, Kaptein EM et al. 1991& Feinstein EI et al.1982).
Clinical relevance of this observation of thyroid dysfunction in patients with nephrotic syndrome is yet to be defined. So far we know such type of observation is lacking in our country. The purpose of the present study is to evaluate thyroid hormone status in patients with nephrotic syndrome in a tertiary care hospital
Materials And Methods
This cross-sectional Observational study was conductedon patients attending in in inpatient and outpatient Department of Rajshahi Medical College Hospital, Rajshahi from April, 2023 to October 2023. A total of 50 patients were selected through purposive sampling. Inclusion criteria: a) Diagnosed patients of Nephrotic syndrome b) Both male & female c) Age more than 18 years d) Participants and / or legally accepted guardians who gave consent and willing to comply with study procedure. Exclusion criteria: a) Secondary nephrotic syndrome. b) Significant renal failure (Serum creatinine > 3 mg / dl). c) Known cases of thyroid disease. d) Systemicillness known to cause thyroiddysfunction eg.-MI, Heart Failure, Chronic liver diseases, Chronic Kidney diseases, Malignancy, Sepsis, Inflammatory condition. General Objective: To assess thyroid status in patient with nephrotic syndrome. Specific Objectives: a) To measure FT3, FT4 and TSH in patient with nephrotic syndrome. b) Level of proteinuria with Thyroid hormone level c) To see socio-demographic data of study population All the data’s were checked and editedafter collection. Then the data were entered into computer and statistical analyses of the results were obtained by using window based computer software devised with Statistical Packagesfor Social Sciences(SPSS-22) with the help of
statistician. The results were presented in tables and figures. In each group, calculation for the continuous variables [mean, standard deviation, no of observations], frequency are calculated in percentage (%). Statistical significance was set at p<0>
Results
This study shows majorityof the patients 27(54.0%) were in the age group 31-40 years followed by 12(24.0%) 20-30 years. The mean age was 32.2±8.5 years.
| Age in years | Frequency | Percentage (%) |
| 20-30 | 12 | 24.0 |
| 31-40 | 27 | 54.0 |
| 41-50 | 3 | 6.0 |
| > 50 yrs | 8 | 16.0 |
| Total | 50 | 100.0 |
| Mean±SD | 32.2±8.5 |
Table 1: Age distribution of the studypatients (n=50)
Out of 50 nephrotic syndrome patients 46.0% were male and 54% were female. The male: female ratio were 1:1.2
| Sex | Frequency | Percentage (%) |
| Male | 23 | 46.0 |
| Female | 27 | 54.0 |
| Total | 50 | 100.0 |
| Male: female ratio | 1:1.2 |
Table 2: Sex distribution of the patients(n=50)
Out of 50 nephrotic syndromepatients 40.0% were normal weight, 12% patients were overweight and 32.0% patients were obese and 16% patients were under weight
| BMI (kg/m2) | Frequency | Percentage (%) |
| Under weight (<18> | 5 | 16.0 |
| Normal (18.5-23) | 20 | 40.0 |
| Overweight (23-25) | 6 | 12.0 |
| Obese (>25) | 16 | 32.0 |
| Total | 50 | 100.0 |
| Mean±SD | 22.98±2.89 |
Table 3: BMI distribution of the patients(n=50)
Out of 50 cases,10.0% were serviceholder, 12% business,16.0% student, 18.0% were farmer and 44.0% patients were housewife.
| Occupation | Frequency | Percentage (%) |
| Service | 5 | 10.0 |
| Business | 6 | 12.0 |
| Laborer | 8 | 16.0 |
| Farmer | 9 | 18.0 |
| Housewife | 22 | 44.0 |
| Total | 50 | 100.0 |
Table 4: Occupational distribution of the study subjects(n=50)
Most patients had UTP between 3.5-10 gm/ 24 hours 45(90.0%) with a mean of 5.96±2.88 gm/24 hours
| Urinary findings | Frequency | Percentage (%) | Mean±SD |
| Urinary output (ml) | |||
| <500> | 3 | 6.0 | |
| 500-1000 | 26 | 52.0 | 992.0±685 |
| 1000-2000 | 14 | 28.0 | |
| >2000 | 7 | 14.0 | |
| Urinary albumin | |||
| ++ | 6 | 12.0 | |
| +++ | 21 | 42.0 | |
| ++++ | 23 | 46.0 | |
| Urinary RBC | |||
| Nill | 14 | 28.0 | |
| <5> | 15 | 30.0 | |
| 5-10 | 7 | 14.0 | 8.2±2.6 |
| 10-15 | 3 | 6.0 | |
| 15-20 | 1 | 2.0 | |
| <20> | 10 | 20.0 | |
| Urinary WBC | |||
| Nill | 8 | 16.0 | |
| <5> | 6 | 12.0 | |
| 5-10 | 14 | 28.0 | |
| 10-15 | 9 | 18.0 | 11.6±3.12 |
| 15-20 | 6 | 12.0 | |
| <20> | 7 | 14.0 | |
| Urinary Cast | |||
| Nil | 25 | 50.0 | |
| + | 11 | 22.0 | |
| ++ | 9 | 18.0 | |
| +++ | 5 | 10.0 | |
| UTP (gm/24 hours) | |||
| <5> | 23 | 46.0 | |
| 5-10 | 22 | 44.0 | 5.96±2.88 |
| >10 | 5 | 10.0 |
Table 6: Biochemical findings in patientswith nephrotic syndrome(n=50)
Low serum FT4 was found in 36(72.0%) of patients and low FT3 was found in 35(70%) of patient with NS. Mean (± SD) TSH was
11.34 (±18.15)in patients with NS. Most of the patient (60%)had elevated TSH among them 25(50%) were between 5-20 mIU/L (sub clinical hypothyroidism) and 5 (10%) were above 20 mIU/L.
| Thyroid hormonelevels | Frequency | Percentage (%) |
| FT4 (nmol/L) | ||
| High (>173) | 0 | 0.0% |
| Normal (54-173) | 14 | 28.0% |
| Low (>54) | 36 | 72.0% |
| Mean±SD 39.34±29.49 | ||
| FT3 (nmol/L) | ||
| High (>3.54) | 0 | 0.0% |
| Normal (1.23-3.54) | 15 | 30.0% |
| Low (<1> | 35 | 70.0% |
| Mean±SD 1.05±0.83 | ||
| TSH (mlu/L) | ||
| High (>20) | 5 | 10.0% |
| Subclinical (5-20) | 25 | 50.0% |
| Normal (<5> | 20 | 40.0% |
| Low(<0> | 0 | 0.0% |
| Mean±SD 11.34±18.15 | ||
Table 7: Distribution of the NS patients by thyroid hormonelevels (n=50)
Table 8 shows ccorrelation between serum albumin and FT4, FT3 and TSH level in nephrotic syndromepatients. Here significant positive correlation between serum albumin with FT4 and FT3 but significant negative correlation with TSH.
Parameters | Pearson correlation test | ||
| r | p | ||
Serum albumin (g/dL) |
FT4 |
+ 0.733* |
<0> |
FT3 |
+ 0.762* |
<0> | |
TSH |
- 0.670* |
<0> | |
Table 8: Correlation between serum albuminand Total T4, T3 and TSH in study patients(n=50)
Pearson's correlation coefficient (r) test was performedto compare relationship between serum albumin and FT4, FT3 and TSH. The test of significance was calculated and p value < 0.05 was accepted as level of significance
Fig. 1: Scatterdiagram showing correlation between serum albumin with total T4

Fig. 2: Scatterdiagram showing correlation between serum albumin with total T3
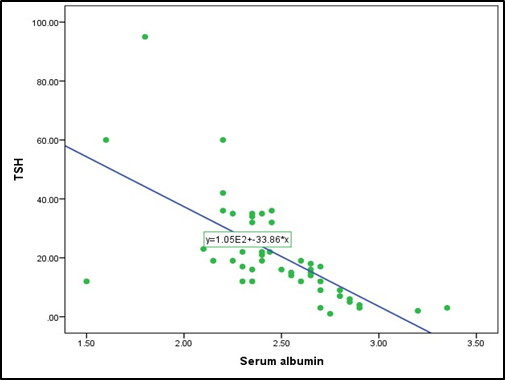
Fig. 3: Scatterdiagram showing correlation between serum albumin with TSH
Discussion
Age of NS variesin different part of the world. In our study highest frequency was observed in age group 31-40 years in patients with NS. The mean age was 32.2±8.5 years. Similar pattern of age distribution was observedby Afrasiabi et al. (1979)and Okpechi et al. (2010). But higher age was noted in patients with NS in Netherlands (mean= 52 years) and Turkey (mean= 47 years) as described by Gilles et al. (2008) and Oguz et al. (2009).Lower age group was found to be affected in other parts of the world as well as in other studies in Bangladesh (Anon 1989, Tarik et al. 2007 and Habib et al. 2012). Although age distribution of patients in our study may not represent the actual patternof age distribution in patients with NS in Rajshahi MedicalCollege Hospital. Because we have taken purposively collected small number of sample after exclusion of certain patient i.e. secondary NS & Significant renal failure. Male-female ratio in our study was 1:1.17. Similarsex ratio was observed by Habib et al. (2012) in patients of same institute and in Africa by Okpechi et al. (2010). Male predominance was observed by Tarik et al. (2007) and also in children by Chowdhury et al. (2010) and Shah et al. (2013). Female predominance also observed in california USA (Afrasiabi et al. 1979).
We had included patient of NS with UTP > 3.5 gm/ 24 hours. In children higher urinary protein was observed (Chowdhury et al. 2010 and Ito et al. 1994) as compared to adults (Oguz et al. 2009 & Afrasiabi et al. 1979) which were more or less similar to our study. Relatively more hypoalbuminemia was also noted among children (Ito et al. 1994, Ahmed et al. 2011 & Chowdhuryet al. 2010) than adults (Oguz et al. 2009, Afrasiabi et al. 1979 & Gilles et al. 2008).
Serum albumin in most of our patient was also relativelyhigher than that found in children. High serum cholesterol was found in all patients in this study. And this finding is invariable as reported by other studies both in adults and children. Proteinuria is associated with urinary excretion of thyroidhormones and thyroxine binding globulin (TBG). Which is a universal finding (Ito et al. 1994, Fonseca et al. 1991, Afrasiabi et al. 1979 & Gavin et al. 1978). Ito et al. (1994) observed that mean serum T4 & T3 to be significantly lower and TSH to be significantly high in children with NS as compared to healthy subjects. After this study
we have agreed upon the above findings but some degree of difference in observation was noted in other studies.i.e. Afrasiabi and colleagues (1979) found only T3 to be significantly lower, at Nijmegen, the Netherlands Gilleset al. (2008) found only TSH to be significantly higher and only lower T4 was noted by Feinstein and others (1982). Unlike others a significantly higher proportion of subclinical hypothyroidism in 20 (50%) patients and overt hypothyroidism in 4 (10%) patients was observed in our study. Among them 10 (25%) had TSH >10. Occurrence of subclinical and overt hypothyroidism was also described by Gilles et al. (2008) but at much lower frequency. In this study significant positive correlation of serum T4 and T3 with serum albumin was seen. Similar correlation was observed by Ito et al. (1994) and Afrasiabi et al. (1979). Similarcorrelation and also a negative correlation between serum albumin and TSH were described by Gilles et al. (2008). Some author did not find any correlation of serum albumin with TSH as does our study. Overt hypothyroidism associated with NS should always be treated. Because overt hypothyroidism is associated with cardiovascular morbidity and mortality. But whether of not subclinical hypothyroidism associated with NS be treated with L-thyroxine remains unclear. Sahni et al. (2014) prospectively studied 35 children with NS who also had subclinical hypothyroidism (mean TSH= 8.93±3.15 μIU/ml) during nephrosis. They found that this thyroid dysfunction resolves after remission of NS (mean TSH= 5.77±0.65 μIU/ml). So Sahni and colleagues concluded that subclinical hypothyroidism state in NS is temporary and improves with remission. So, no treatment is needed. On the contrary a trial of low dose L-thyroxine in 59 patient with primary NS and thyroid dysfunction done by Anon (2010) shown that small doses of L-thyroxine can influence remission of NS, in terms of shortening the course and improving the cure rate.
Moreover, studies have described that thyroxine replacement can slow the decline in renal function in CKD patient with subclinical hypothyroidism. Shin et al. (2013) was studied 113 patient with stage 2-4 CKD and subclinical hypothyroidism and found statistically significant impact of thyroid hormone on the decline in eGFR. They found that the declinein eGFR were significantly attenuated by L-thyroxine supplement in both stage 2 and stage 3-4 CKD patients. It was also observed that thyroid hormone replacement therapy had delayed reachingCKD stage
5 within 10 years in 81.1% of CKD patients with subclinical hypothyroidism. In addition to Kreisman et al. (1999) and others Connor & Taylor (2008) reported two cases of reversible renal impairment secondary to hypothyroidism. Patients were found to have hypothyroidism and reduced GFR. Other causes of renal impairment were excludedand renal biopsyshowed no feature of GN. Replacement with L-thyroxine brought about complete recovery of renal function. Behind this, three mechanisms were suggested- (1) reduction of GFR due to low cardiac outputand renal blood flow, (2) Thyroxine may mediate tubular secretion of creatinine and (3) Hypothyroidism may increase creatinine release from muscle. A similar case was reported by Asim and Esnawi (2010). So hypothyroidism can cause renal impairment in the absence of glomerular disease. L-thyroxine replacement can reverse the renal impairment in patients without glomerular disease. Thyroxinecan also slow the progression of CKD and delay the onset of ESRD in patient with stage 2-4 CKD with subclinical hypothyroidism. Therefore, Shin et al. (2013) concluded that thyroid hormone treatment should be considered in patients with renal impairment and subclinicalhypothyroidism.
Clear benefit of thyroxine replacement was observed in case of patients with CKD whereas controversial results were found in case of ARF (Acker et al. 2000).
Considering the effect of L-thyroixine on progression of CKD observed by several researchers we can say that, L-thyroxine replacement may be of benefitin patients with NS and subclinical hypothyroidism who also have renal impairment. But larger randomized controlled trial will be necessary to recommend L- thyroxine replacement in this patient group without renal impairment.
Conclusion
Thyroid hormones are necessaryfor the growth and development of kidney. On the contrarydifferent form of kidney diseases are associated with various types of thyroid dysfunctions. This association between renal disease and thyroid dysfunction has been known for years. In patientswith nephrotic syndrome (NS) large amount of protein is lostin urine along with thyroid hormonesand hormone bindingproteins. This hormone loss may lead to low FT4, FT3 and sometimeshigh TSH level. NS was found to be associated with subclinical and overt hypothyroidism. We have systematically evaluated patients with NS to see the frequency and pattern of thyroid dysfunctions in patients with NS. Considering the result of this study and observations done by other researchers it can be concluded that nephrotic range proteinuria is associated with thyroid hormone loss in urine and this can lead to significant reduction inserum FT4, FT3 and significant increase in serum TSH. Patients with NS can develop subclinical hypothyroidism or even overt hypothyroidism. Apparently it is relatedto the degree of proteinuria and also to the thyroid reserveof the patient as not all patients with reduced serum FT4 and FT3 has raised TSH level. It was found that L-thyroxine replacement can improve outcome in patients with subclinical hypothyroidism and renal impairment. But despite tremendous influence of thyroid hormone over renal function its role in NS remains unclear.
Limitation:
It is a smallscale cross sectionalstudy. So to decide whetheror not we screen all the patientwith NS a large scale study should bedone. Other limitations of our study are patient and healthy individuals are not screened for auto-immune thyroid disease (ie. Anti-TPO antibody) and fT4 & fT3 were not measured.
References
-
Acker CG, Singh AR, Flick RP, Bernardini J, Greenberg A, Johnson JP, 2000. A trial of thyroxine in acute renal failure. Kidney International, 57:293-298.
Publisher | Google Scholor -
Adlkofer F, Hain H, Meinhold H et al, 1983. Thyroid function in patients with proteinuria and normal or increased serum creatinine concentration, Acta Endocrinol, 102, 367-76
Publisher | Google Scholor -
Afrasiabi MA, Vaziri ND, Gwinup G et al, 1979. Thyroid function studies in the nephrotic syndrome, Ann Int Med, 90, 335- 8.
Publisher | Google Scholor -
Ahmed F, Prasad SK, Afroz S, Chowdhury K, Quader, Hanif M, 2011. Low serum IgG level during remission: A predictor of frequent relapse nephrotic syndrome. DS (Child) HJ, 27 (2):64-67.
--> -
l-Fifi S, Girardin C, Sharma A, Rodd C,1999. Moderate renal failure in association with prolonged acquired hypothyroidism in children. Acta Paediatrica, 88:715-717.
Publisher | Google Scholor -
Asim M, Esnawi ME, 2010. Renal dysfunction manifesting in subclinical hypothyroidism- a possible role of thyroxine. NDT plus, 3:282-284.
Publisher | Google Scholor -
Bagga A, Mantan M, 2005. Nephrotic syndrome in children. Indian J Med Res, 122, 13-28.
Publisher | Google Scholor -
Brohee D, Delespesse G, Debisschop MJ, Bonnyns M, 1979. Circulating immune complexes in various thyroid diseases. Clinical and Experimental Immunology, 36:379-383.
Publisher | Google Scholor -
Busko M, 2013. Function decline in CKD with SCH. Thyroid, 23:654-661.
--> -
Capasso G, De Tommaso G, Pica A, Anastasio P, Capasso J, Kinne R, De Santo NG, 1999. Effects of thyroid hormones on heart and kidney functions. Mineral and Electrolyte Metabolism, 25(1-2):56–64.
Publisher | Google Scholor -
Chadban S J, Briganti E M, Kerr P G, D W Dunstan, Welborn T A, Zimmet P Z, Atkins R C, 2003. Prevalence of Kidney Damage in Australian Adults: The AusDiab Kidney Study. J Am Soc Nephrol, 14, S131–S138.
Publisher | Google Scholor -
Chadha V, Alon US, 1999. Bilateral nephrectomy reverses hypothyroidism in congenital nephrotic syndrome. Pediatr Nephrol, 13:209-211.
Publisher | Google Scholor -
Chowdhury EUA, Huq MN, Jaigirdar MA, 2010. Pattern of Nephrotic Syndrome in Children Admitted in Bangladesh Medical College Hospital. Bangladesh Med Coll J, 15(2):67-73.
--> -
Connor A, Taylor JE, 2008. Renal impairment resulting from hypothyroidism. NDT plus, 6:440-441.
Publisher | Google Scholor -
Dagan A, Cleper R, Krause I, Blumenthal D, Davidovits M, 2011. Hypothyroidism in children with steroid-resistant nephrotic syndrome. Nephrol Dial. Transplant. doi: 10.1093/ndt/gfr665.
Publisher | Google Scholor -
Den Hollander JG, Wulkan RW, Mantel MJ, Berghout A, 2005. Correlation between severity of thyroid dysfunction and renal function. Clinical Endocrinology, 62:423–427.
Publisher | Google Scholor -
Feinstein EI, Kaptein EM, Nicoloff JT, Massry SG, 1982. Thyroid function in patients with nephrotic syndrome and normal renal function. American Journal of Nephrology, 2:70–76.
Publisher | Google Scholor -
Fonseca V, Thomas M, Katrak A, Sweny P, Moorhead JF, 1991. Can urinary thyroid hormone loss cause hypothyroidism? Lancet, 338(8765):475-476.
Publisher | Google Scholor -
Fonseca V, Thomas M, Katrak A, Sweny P, Moorhead JF, 1991. Can urinary thyroid hormone loss cause hypothyroidism? Lancet, 338, 475-6.
Publisher | Google Scholor -
Gavin LA, Mcmahon FA, Castle JN, Cavalieri RR, 1978. Alterations in serum thyroid hormones and thyroxine-binding globulin in patients with nephrosis. J Clin Endocrinol Metab, 46:125-130.
Publisher | Google Scholor -
Gilles R, den Heijer M, Ross AH, Sweep FCGJ, Hermus ARMM, Wetzels JFM, 2008. Thyroid function in patients with proteinuria, Netherlands the journal of Medicine, 66(11):483-485.
Publisher | Google Scholor -
Goddard J, Turner AN, Stewart LH, 2010. ‘Kidney and urinary tract disease’, Colledge NR, Walker BR, Ralston SH (eds), Davidson’s Principles and Practice of Medicine. 21st ed, Elsevier, Churchill Livingstone, pp.481.
--> -
Habib M A, Badruddoza S M, 2012. Pattern of glomerular diseases among adults in Rajshahi, the Northern Region of Bangladesh. Saudi J Kidney Dis Transpl, 23(4):876-880.
Publisher | Google Scholor -
Iglesias P, Díez JJ, 2009. Thyroid dysfunction and kidney disease. European Journal of Endocrinology, 160:503-515.
Publisher | Google Scholor -
Ito S, Kano K, Ando T, Ichimura T, 1994. Thyroid function in children with nephrotic syndrome. Pediatr Nephrol, 8:412-415.
Publisher | Google Scholor -
Iwazu Y, Nemoto J, Okuda K et al, 2008. A case of minimal change nephrotic syndrome with acute renal failure complicating Hashimotoâs disease, Clin Nephrol, 69, 47-52.
Publisher | Google Scholor -
Junglee NA, Scanlon MF, Rees DA, 2006. Increasing thyroxine requirements in primary hypothyroidism: don’t forget the urinalysis, Journal of Postgraduate Medicine, 52(3):201–203.
Publisher | Google Scholor -
Kaptein EM, Feinstein EI, Massry SG, 1982. Thyroid hormone metabolism in renal diseases, Contributions to Nephrology, 33, 122–35.
Publisher | Google Scholor -
Kaptein EM, Hoopes MT, Parise M, Massry SG, 1991. rT3 metabolism in patients with nephrotic syndrome and normal GFR compared with normal subjects. American Journal of Physiology, 260:E641–E450.
Publisher | Google Scholor -
Khan A, Khan MMA, Akhter S, 2002. Thyroid disorder, eitiology and prevalence. J Med Sci, 2(2):89-94.
Publisher | Google Scholor -
Kreisman SH, Hennessey JV, 1999. Consistent reversible elevations of serum creatinine levels in severe hypothyroidism. Archives of Internal Medicine, 159:79-82.
Publisher | Google Scholor -
Lane JC, Langman CB, Finberg L, Spitzer A, Windle ML, 2014. Pediatric nephrotic syndrome. Available from: http://emedicine.medscape.com/article/982920-overview.
--> -
Liappis N, Rao S, 1985. Behavior of the levels of free triiodothyronine, triiodothyronine, free thyroxine, thyroxine, thyrotropin and thyroxinebinding globulin in the serum of children with nephrotic syndrome, Klin Padiatr, 197, 423-6. 34. Mariani LH, Berns JS,2012. Renal menifestations of thyroid
Publisher | Google Scholor -
Mariani LH, Berns JS,2012. Renal menifestations of thyroid disease. J Am Soc Nephrol, 23:22-26.
--> -
McLean RH, Kennedy TL, Rosoulpour M, Ratzan SK, Seigel NJ, Kauschansky A, Genel M, 1982. Hypothyroidism in the congenital nephrotic syndrome. J Pediatr, 101:72-75.
Publisher | Google Scholor -
Menon VU, Sundaram KR, Unnikrishnan AG, Jayakumar RV, Nair V, Kumar H, 2009. High prevalence of undetected thyroid disorders in an iodine sufficient adult south Indian population. J Indian Med Assoc, 107(2):72–77.
Publisher | Google Scholor -
Montenegro J, Gonzalez O, Saracho R, Aguirre R, Gonzalez O, Martinez I, 1996. Changes in renal function in primary hypothyroidism. American Journal of
Publisher | Google Scholor -
Mooraki A, Broumand B, Neekdoost F, Amirmokri P, Bastani B, 2003. Reversible acute renal failure associated with hypothyroidism: report of four cases with a brief review of literature. Nephrology, 8:57-60.
Publisher | Google Scholor -
Nephrotic syndrome associated with thyroid dysfunction in clinical observation. 2010. clinical medicine papers. Available from: http://www.eng.hi138.com/.../clinical.../240939_nephrotic- syndrome-associated-with- thyroid-dysfunction-in-clinical- observation.asp
--> -
Oguz Y, Yilmaz MI, Acikel C, Eyileten T, Caglar K, Oktenli C, Yenicesu M, Vural A, 2009. The relationship between adiponectin levels and degree of proteinuria in patients with nephrotic and non-nephrotic proteinuria. Renal Failure, 31:29- 35.
Publisher | Google Scholor -
Okpechi IG, Rayner BL, 2010. Nephrotic Syndrome in Adult Black South African: HIV-Associated Nephropathy as the Main Culprit. Journal of National Medical Association, 102(12):1193- 1197.
Publisher | Google Scholor -
Renal disease, Kumar P, Clark M (eds), 2009, Kumar & Clark’s Clinical Medicine. 7th ed, Saunders-Elsevier, Edinburgh, pp.584-592.
Publisher | Google Scholor -
Sah JP, Pandey R, aiswal S, Sharma B, Chaudhury SS, 2013. Correlation of hypoproteinemia and hypoalbuminemia with hypercholesterolemia in the children with nephrotic syndrome. STM Journals, 3(2):1-11. 44. Shahni V, Nanda S
Publisher | Google Scholor -
Sharpstone P, Ogg CS, Cameron JS, 1969. Nephrotic syndrome due to primary renal disease in adults: 1. Survey of incidence in south-east England, Br Med J, 2(5656), 533-5.
Publisher | Google Scholor -
Shin DH, Lee MJ, Lee HS, Oh HJ, Ko KI, Kim CH, Doh FM, KOO HM, Kim HR, Han Jh, Park JT, Han SH, Yoo TH, Kang SW, 2013. Thyroid hormone replacement therapy attenuates the decline of renal function in chronic kidney disease patients with subclinical hypolthyroidism. Thyroid, 23(6):654-661.
Publisher | Google Scholor -
Tarik MH, Ekram ARMS, Haque MA, Islam AKMM, Uddin MJ, 2007. Renal Pathology in Adult Onset Idiopathic Nephrotic Syndrome: A Study of 100 cases. TAJ, 20(2):140-143.
Publisher | Google Scholor -
Trouillier S, Delèvaux I, Rancé N, André M Voinchet H, Aumaître O, 2008. Nephrotic syndrome: don’t forget to search for hypothyroidism. Rev Med Interne, 29 (2):139-144.
Publisher | Google Scholor -
4Uddin MJ, Alam KM, Mohammed FR, Alam MB, 2009. Hypothyroidism and nephrotic syndrome- A rare association. J Medicine, 10:34-35.
Publisher | Google Scholor -
Unnikrishnan AG, Kalra S, Sahay RK, Bantwal G, John M, Tewari N, 2013. Prevalence of hypothyroidism in adults: An epidemiological study in eight cities of India. Indian J Endocrinol Metab, 17(4):647-652.
Publisher | Google Scholor -
Unnikrishnan AG, Menon VU, 2011. Thyroid disorder in India: An epidemiologic perspective. Indian J Endocrinol Metab, 15(supp 2):S78-S81.
Publisher | Google Scholor -
Villabona C, Sahun M, Roca M, Mora J, Go´mez N, Go´mez JM, Puchal, Soler J, 1999. Blood volumes and renal function in overt and subclinical primary hypothyroidism. American Journal of the Medical Sciences, 318(4):277–280.
Publisher | Google Scholor -
Yoshida K, Sakurada T, Kaise K, Kaise N, Nomura T, Itagaki Y, Yamamoto M, Saito S,Yoshinaga K, 1988. Measurement of thyroid stimulating hormone (TSH) in human urine. Endocrinol Japan, 35(5):733-9.
Publisher | Google Scholor -
Karanikas Gm Schütz M, Szabo M, Becherer A, Wiesner K, Dudczak R. Kletter, 2004. Isotopic renal function studies in severe hypothyroidism and after thyroid hormone replacement therapy. American Journal of Nephrology, 24:41-45
Publisher | Google Scholor
