Review Article
1luisetto m independent researcher , applied pharmacologist , hospital pharmacist manager , italy 29121 PC area Italy
2Edbey k. Professor of physical chemistry Libian authority for scientific reasearch
3Mashori G.R. professor departement of medical and health sciences for woman, Peoples university Pakistan
4Cabianca L medical laboratory Turin , citta' della salute -italy
5latyshev O.YU IMA academy president
*Corresponding Author: Luisetto M, independent researcher, applied pharmacologist, hospital pharmacist manager, italy 29121 PC area Italy
Citation: Luisetto M, Almukthar N, Edbey K., Mashori G.R. Mashori G.R., Cabianca L., and Latyshev O.YU, Oral Drops as pharmaceutical form and its Peculiarity in Galenic Practice, Pharma Scope and Advances in Drug Sciences, vol 1(1). DOI: https://doi.org/10.64347/3066-2583/PSADS.004
Copyright: © 2024, Luisetto M, this is an open-access article distributed under the terms of The Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Received: October 29, 2024 | Accepted: November 04, 2024 | Published: November 21, 2024
Abstract
Aim of this work it to verify the relevant concept useful in galenic practice related the pharmaceutical form: galenic oral drops.
Due by its specificity are analyzed the critical properties to be considered: some times are used high concentration of very active APIs and it is needed to verify the chemico physical characteristics involved as well as the need to use calibrated dropper to avoid overdosage (needed verify of the assessment dosage).
Using Different vehicle, it can change the number of drops for milliter.
A specific focus on the excipients is provided and the quality control required by Pharmacopeia.
Some formulation in use will be reported to clarify the concept.
Keywords: galenic oral drops, pharmacy, chemico-physical properties, excipients, vehicle Dropper, safety, quality control, Pharmacopeia, NBP.
Introduction
Galenic oral Drops are liquid preparations of APIs, multidose, usually in solution, produced in the galenic pharmacy lab to be administered to patients in small volumes with specific measuring devices (are needed calibrated droppers).
This makes possible to the physicians to prescribe doses adapted to the single patients need.
This pharmaceutical form include: oral drops, nose and ear drops (instillations) and also eye drops. This can be solution, suspension or emulsion. Eye drops are generally made in water-based carriers. This must to be aseptic and isotonic, with breast secretions, buffer and white no foreign bodies, in order to avoid eye irritation. Nasal drops are drops of drug solution into the nose with a specific dropper.
Figure: 1 glass bottle and related droppers
The mass or volume of a single drop depends on the kind of droppers and its diameter of spill (3 mm) and related the vehicle used (water bases, alcoolic solution or oil), the superficial tension of the liquid (API plus vehicle), density, viscosity.
Example of oral drops:
1)water solution based (codeine, potassium iodide);
2)idroalcoolic based (ephedrine drops)
3)Idrogliceric -alcoholic solution (digoxin oral drops)
4) idrogliceric (ergometrin drops).
Of interest also the fitotherapic fluid extracts mixture.
For Water solutions: 20 drops =1 ml. The oral dropper according Italian official pharmacopeia FU IX Vol I,: "20 drop of water at temperature of 20 + - 1° C ,dripped at free fall from a normal dropper maintained in vertical position with a kostant flux of 1 drop by second , must weigh 1.0 g (+/- 0.05)”.
Other method: 20 drops = 1 ml.
(Instead, the Alcoholic solution: 50 drops = 1 ml)
Limits alcohol etilic for OTC (FDA): no more the 0,5% under 5 years, 5% for 5-10 year and 10% > 12 year
Often, they are oral drops are aqueous solutions rather than oily. The vehicle to be used vary according the need so API solubilization or to get right stability. Also, the pressure used in the top of the dropper can influence the drop diameter. The nasal drops shall be isotonic with a neutral pH and viscosity similar to that of nasal secretions. Ear drops are drops of a drug solution that are piped into the ear. These are commonly used to clean ears, soften wax and treat mild infections. Related oral drops: can be used also cosolvent to make easier the solution if water low soluble APIs: (alcohol of specific gradation, glicerin, propilen glicol.)
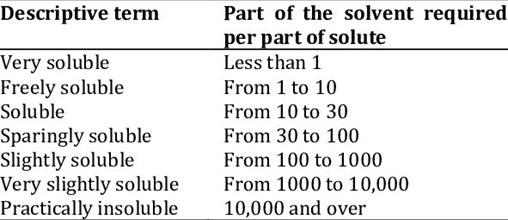
Figure 2: USP and BP solubility criteria
Generally, this are diluted in water before to be subminiaturized (unitary doses: 1- 2 ml).
Between the excipients are in use: suspending agents (for suspension), PH regulator, antiseptics, antioxidants, edulcorates.
If very low water solubility of the API it is needed to use cosolvents: idroalcolic solution of determinate gradation or glycerin solution. (For some APIs this can increase the stability preventing degradation). It is needed then to verify the complete solubilization of the API.
About the technological operation needed it is possible to see: mortar and pestle to crash API (cp if used) or to mix the pure API required with solid excipients, Dissolution (if solution), mixing, heating if API is not term sensible, preparation of the suspension- emulsion (ore using ready for use vehicle), filtering if needed. The solubilization is increased with mixing well.
So are crucial the chemico physical characteristics of the APIs to be used as well as the excipients or vehicle needed: solubility, thermostability, HLB profile of suspending agents, viscosity, chemico compatibility at PH need, instability during the time and with solvent used, use of cosolvents, preservants use and its effect of the final preparation.
After the preparation the label must report % w/vol and written information about how many drops are the same for the required unitary doses to be taken.
Doses and uniformity of doses quality control: in a graduated little cylinder put the number of drops to be single subministrated, then weight, after this add equal number of drops and weigh again and so on: no single mass must to differ more than 10% and the global mass of the ten measures must not overcome15%.
In order to better explain some concepts, it is useful to see some formulation is use:
lormetazepam oral drops MG form technical sheet: 20 Ml 2,5 Mg/ml. 1 ml di sol contains 2,5 mg of lormetazepam. Generally single dose for adult: 1-2mg (1 mg equivalent to 10 drops).
excipients: mannitol, ethanol, depurated water.
Stability: see the pharmacopeia NBP for oral liquid formulation, literature or other like the preservants use in current practice.
For non-aqueous liquid formulation (oil, glycerite), liquids with alcolic content not inferior to 25% (like tincture).
The limits are calculated considering the component of the preparation with the shorter expiry data: the period authorized for use is 25% of the residual validity time. In every way not can be overcome the limits of 6 month.
For all other preparation (solution) the limits are 30 days.
Materials and Methods
Tests- assay according Pharmacopeia XII ed for ORAL LIQUID:
- uniformity of dose unit
- uniformity of content
- mass uniformity
- mass uniformity of doses released by multidose containers
- Doses and uniformity of doses for oral drops.
Other of formulation:
Codeina Drops FUVIII (opioid pain reliever which is used to treat mild to moderately severe pain and to help reduce coughing).
CODEIN FOSFATE G 2
NIPAGINA G 0,1
H2O DEP QB 100 ML
Digoxin oral drops: used in several cardiac pathologies such as atrial fibrillation and heart failure
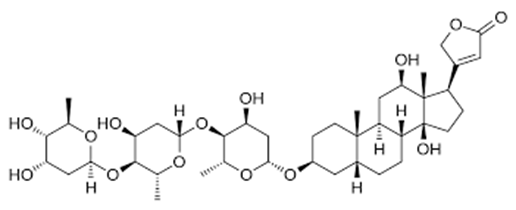
Figure 4: digoxin
Digoxin greater than 10ml 0,5mg/ml, (10 ml of etilic alchool at 70%) as restored drug.
warning: pay attention in children < 10> 10 to the maximum doses of ETOH admitted
According article Curr Ther Res Clin Exp. 2014
Ethanol Pharmacokinetics in Neonates and Infants
Elizabeth Marek et al,
“The FDA has set a maximum limit of 0.5% ethanol in oral OTC products intended for children younger than age 6 years (21 CFR P328 2013). The American Academy of Pediatrics Committee AAPC on Drugs has recommended that the amount of ethanol contained in any preparation should not be able to produce a blood concentration >25 mg/dL after a single dose, whereas the EMA in recent time proposed that blood ethanol levels should not exceed 1 mg/dL after a single dose (or a dose of 6 mg/kg) in children younger than age 6 years.”
Digoxin greater than 10ml 0,5mg/ml, (10 ml of etilic alchool at 70%) as restored drug.
warning: pay attention in children < 10> 10 to the maximum doses of ETOH admitted
According article Curr Ther Res Clin Exp. 2014
Ethanol Pharmacokinetics in Neonates and Infants
Elizabeth Marek et al,
“The FDA has set a maximum limit of 0.5% ethanol in oral OTC products intended for children younger than age 6 years (21 CFR P328 2013). The American Academy of Pediatrics Committee AAPC on Drugs has recommended that the amount of ethanol contained in any preparation should not be able to produce a blood concentration >25 mg/dL after a single dose, whereas the EMA in recent time proposed that blood ethanol levels should not exceed 1 mg/dL after a single dose (or a dose of 6 mg/kg) in children younger than age 6 years.”
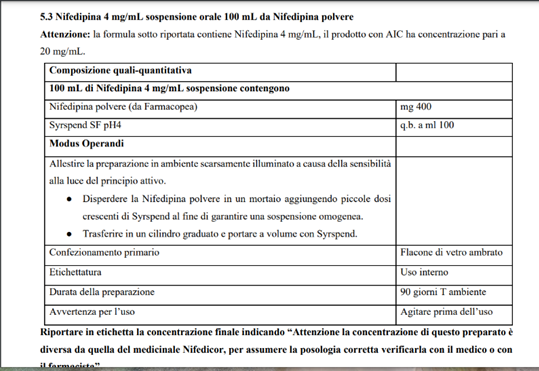
Figure 4: SIFAP procedure NIFEDIPIN oral suspension Calcium Blocker; is used to treat high blood pressure and to control angina (chest pain)
Acetic acid ear drops:
Acid Acetic medicinal 2%
Alcohol etilic 70° qb 30 ml
Warning: medicinal acetic acid is a solution at 33% of glacial acetic acid diluted in deputed water. Glacial acetic acid is more irritant vs the use of the medicinal acetic acid due its greeter concentration
Lorazepam oral drops: for short-term relief of anxiety symptoms
Lorazepam 200 mg
Mannite 800 mg
Ethanol 70 ml
Depurated water qb 100 ml
The mannite is used to correct the taste, and ethanol act also as preservant.
Procedure: weigh the API needed, dissolve lorazepam in alcohol and mannite in water then mix these two solutions, add final water since total volume requested.
Control: the amount to be dispensed, aspect, organoleptic characteristics, dose and uniformity of oral drops

Figure 5: Other BDZ
Hydromorphone HCl 10 mg/mL Oral Drops: a potent opioid analgesic formulated for the management of severe pain requiring continuous opioid treatment when alternative options are inadequate.

Figure 6: idromorphon, for severe pain control
Cannabis: OIL Bediol (from cannabis sativa) 1: 10 relationship (drug vs oil) 5 gr cannabis into 50 ml, grinding, add of olive oil Ph EU, heat at 98 centigrade for 120 minutes mixing, cooling, filtration under pressure.
The cannabis contains inactive molecule like THCA acid that need to be activated thought decarbossilation.
Mandatory in Italy to verify the title in mg / ml with HPLC- MS of GC-MS in analytical lab. And to give to the caregiver or patients the certificate of analysis with the title.
The label must report “under narcotics normative rules”
Posology: initial dose 6 sublingual drops in the evening for 7-10 days then gradually increase according medical prescription.
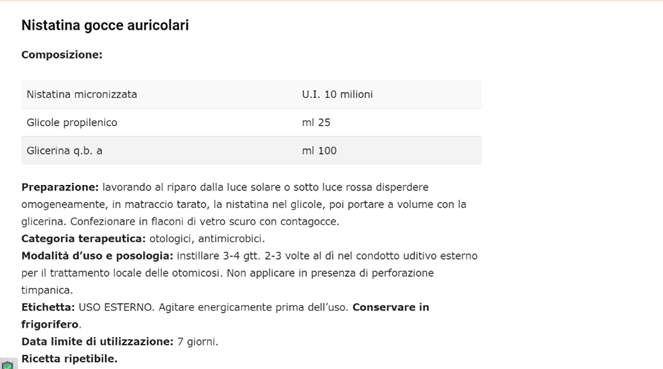
Figure 7: From https://www.farmacianews.it/gocce-auricolari/ NISTATIN ear drops for some fungal infections
According V. P. Sobolev et al about silver proteinate nasal drops:
“The drug is a silver proteinate agent with broad antibacterial anti-inflammatory activity. The drug is used to treat the infectious rhinitis.”
Niaouli essence 2% nasal drops:
Balsamic, expectorant, anti-catarrhal and antiseptic in upper respiratory tract inflammation, particularly in nasal congestion.

Figure 8: Niauli
Other examples
Pilocarpine HCl 2 mg/ml Oral Drops, this medication is used to treat conditions such as glaucoma, xerostomia, Sjogren's Syndrome, Keratoconjunctivitis Sicca, and ocular hypertension.
In galenic field for the magistral formula are required specific final control in order to guarantee efficacy and safety:
- verify of the right procedure followed by the pharmacist
- aspects of the final product prepared, if emulsion or suspension verify the separation of phases if present. (If suspension verify that the precipitate is easy to be resuspended with simply shaking).
- verify the held of the primary closing
- right labeling verifies with also modality conservation.
- mass uniformity to be performed in a sample (the dimension depends on the number of doses form. No one must to move away from +10/-10 % of the medium weigh (pharmaceutical form at unique doses).
- the amount to be dispensed
- absence of visible particle and if needed pH.
Related the microbiological requirement: it must to be followed the pharmacopeia rules for oral liquid formulation.
According Italian pharmacopeia XII ed: Table n. 8: maximum doses for adults, beyond this value the pharmacist must not dispense the prescription unless specific physician written declaration
(For pediatric patients see literature or other therapy textbook or guideline)
Material and methods
With an observational point of view some relevant literature, textbook or pharmacopeia are reported and analyzed related the topic of this article. Various figure (1-10) is submitted to clarify some concepts. An experimental project is reported and after analyzing all this a global conclusion s provided.
Result
Form literature
by Carmen-Maria Jîtcă et al
“The EMA also issued a guideline on the development of medicines for pediatric use. In this guideline, separate sections with instructions for liquid drug formulations are provided, like as oral suspensions OS and oral drops, and effervescent, soluble, and dispersible preparations, including considerations regarding packaging and measuring devices in order to avoid dosing errors”. (1)
EMA/CHMP/QWP/805880/2012 Rev. 2 2013 Committee for Medicinal Products for Human Use (CHMP)
Pediatric Committee (PDCO) Guideline on pharmaceutical development of medicines for pediatric use”. In order to avoid counting errors, alternative measuring devices should be considered where the dose comprises more than 10 drops.
Unless otherwise justified, oral drops OD will only be considered acceptable for pediatric medicines containing active substances with a wide therapeutic window.
The volume dispensed (drop size) will be determined by the design and physical characteristics of the dropper, the chemical- physical properties of the liquid and how the dropper is handled. Clear instructions should be included in the SmPC and PIL about the correct use of the dropper” (2)
According Giuliana Cangemi et al related the Titillation of Oil (THC -CDB) “the analytical phase included the development and validation of the analytical method Ultra High-Performance Liquid Chromatography coupled to the tandem mass spectrometry, (UHPLC-MS/MS) in the 2 laboratories, with common procedures and the comparison of results conducted both on reference material and real samples of olive oil galenic preparations” (3)
Alessandra Manca et al about the composition of some Cannabis products “Their contents in Δ−9-THC and CBD are 22% THC and ˂1?Dfor Bedrocan®, 6.5% and 8% for Bediol®, < 1>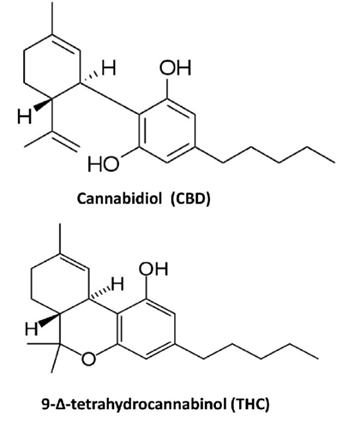
Figure 9: CBD and THC chemical formula
Ribes nigrum gliceric macerate 2- 10 %: oral drops, alimentary integrator Ribes nigrum L. extract, fresh gems in glycerin, alcohol and water.
Afaf Ejaz et al
“Various bioactive present in blackcurrants (Ribes nigrum) have different functional and pharmacological aspects including various anti-inflammatory, antioxidant, and antimicrobial properties. The most the important bioactive include anthocyanins, flavanols, phenolic acids, polyunsaturated fatty acids” (5)

Figure 10: Ribes nigrum MG oral drops

Figure 11: ribes nigrum
“Neonates may be more susceptible to drug–excipient or excipient–excipient pharmacokinetic interactions (PKI)when compared with adults because of their reduced metabolic activity (7).
Experimental experience
In order to verify the tartare of the dropper for oral dops: for water solution verify that the below opening of the dropper is 3 mm the position of the dropper must to be in vertical 1-2 drops by second. Collect 20 drops using the dispenser Measure the volume or weight.
Discussion
Before to start the preparation in the galenic laboratory is needed to verify the chemico physical properties of the API in order to choose the vehicle or excipient to be used (generally are written in the physician prescription or in officinal formula). Useful to observe literature, other formulation in use, pharmacopeia, textbook of technical pharmacy and other relevant.
Because oral liquid drops are solution or suspension or emulsion in the preparation it must to be prepared following the technological indication for each of this specific pharmaceutical forms as reported in various technical pharmaceutical discipline (or galenic laboratory).
The water solubility of the API is a driver to choose the formulation (cosolvents to be used, or suspending agents). The excipients to be used are the excipients for oral liquid s reported in technical pharmacy textbooks. If used alcohol take in consideration the limits for children to avoid the toxicity. Preservants can be used to increase the expiry time if according literature.
If suspension is mandatory to label with “shake before use “and if used narcotics to add the phrase in the label “according narcotics rules “
The same in the label it must to be reported if used doping APIs or excipients with this characteristic. Crucial to verify the tartare of the dropper in the galenic lab and the right labeling for the safety of the patients.
Conclusion:
Related the pharmaceutical form: oral drops it is mandatory to consider that sometimes the APIs use are very active and high concentrated.
- For this reason, it is crucial to verify the subminitration using calibrated droppers.
- The excipients to be used must to be able to adequately solubilize the APIs using also preservants if needed.
- The excipient to be used must to be compatible with the drug and in ?mitted for the specific use.
- It is necessary that the label report the number of drops to have the unitary dosage needed (mg of the APIs)
- (The number of get for unit dosed prescribed to be taken and the % w/v or w/w).
Mandatory to follow the pharmacopeia requirement related the quality control in galenic lab, the expiry date to prevent microbiological contamination or API inactivation.
The right labeling with all required by normative rules is crucial as well as the right patient information for the use.
Conflicts of Interest:
The author declares no conflicts of interest.
References
-
Stability of Oral Liquid Dosage Forms in Pediatric Cardiology: A Prerequisite for Patient’s Safety—A Narrative Review Carmen-Maria Jîtcă,George Jîtcă,Bianca-Eugenia Ősz,A.Pușcaș,Silvia Imre Pharmaceutics 2023, 15(4), 1306.
Publisher | Google Scholor -
Committee for Medicinal Products for Human Use (CHMP) Paediatric Committee (PDCO) Guideline on pharmaceutical development of medicines for paediatric use
Publisher | Google Scholor -
Titolazioni dei preparati galenici oleosi a base di cannabis in Regione Liguria: progetto sperimentale dei laboratori di riferimento regionale Giuliana Cangemi, Paolo Bucchioni, Gino Tripodi, Sebastiano Barco, Paolo Franceschini Isa Mavi Sbarbaro,Gianfranco Petricciani, L.Barbagallo, Angelo Maffia, Eugenia Livoti, Clara Cannas, Eleonora Russo, Barbara Rebesco, Flavia Lillo
--> -
A description of Cannabinoid levels in Cannabis oil by high-performance liquid chromatography-mass spectrometry in a reference laboratory of North-Italy Alessandra Manca, Alice Palermiti, Jacopo Mula, Elisa Delia De Vivo, Sandra Zeaiter, Marco Simiele, Amedeo De Nicolò, M. Cantù, Jessica Cusato, Antonio D'Avolio
Publisher | Google Scholor -
Biological activities, therapeutic potential, and pharmacological aspects of blackcurrants (Ribes nigrum L): A comprehensive review Afaf Ejaz, Sadaf Waliat, Muhammad Afzaal, Farhan Saeed, Aftab Ahmad, Ahmad Din, Huda Ateeq, Asma Asghar, Yasir Abbas Shah, Ahmad Rafi, M. Rahman Khan DOI: 10.1002/fsn3.3592
Publisher | Google Scholor -
Nov 2020 V. P. Sobolev, V. M. Svistushkin, M. G. Leyzerman, K. R. Magomedov, D. B. Bidanova, G. N. Budagova, The efficacy of silver proteinate in the treatment of acute nasopharyngitis Meditsinskiy sovet = Medical Council. DOI: 10.21518/2079-701X-2020-16-43-49
--> -
Ethanol Pharmacokinetics in Neonates and Infants, Elizabeth Marek, W. K. Kraft, Volume 76, Dec 2014 Current Therapeutic Research.
--> -
E. Ragazzi Galenica pratica edit. Cortina 2006.
--> -
Procedure Gestionali e Tecnologiche per il Laboratorio Galenico della Farmacia APPA Project P. Brusa, A Germano 2008.
--> -
BNF for children 2023 -24.
--> -
Table 8 FU XII IT official pharmacopeia and NBP
--> -
DM 18 November 2003 Italy; Procedure for preparation of magistral and officinal.
--> -
Handbook of Pharmaceutical Excipients – Ninth Edition Edited by Paul J Sheskey, Bruno C Hancock, Gary P Moss, David J Goldfarb.
Publisher | Google Scholor
